 Hashimotos Thyroid Disease and Molecular Mimicry
Hashimotos Thyroid Disease and Molecular Mimicry
by Jeffrey Dach MD
Hashimotos Thyroid Disease, What is It ?
Originally described by a Japanese surgeon in 1912, Hashimotos is an autoimmune disease of the thyroid gland.(30) Our own immune system attacks the thyroid gland causing inflammation and eventual destruction of the thyroid gland.(1) In the final stages, there is loss of thyroid function and hypothyroidism requiring thyroid replacement with thyroid pills which restores thyroid levels.
What causes the auto-immune attack in Hashimotos ?
The cause of auto-immune thyroid disease is not completely known by medical science. However, a combination of genetic and environment factors are thought to play a role.(30)
“Molecular Mimicry” is the new theory which explains how our immune system can be “tricked”into attacking the thyroid cells.(1-5) A bacteria organism called Yersinia which resides in our intestinal bacteria has been found to share identical amino acid sequences when compared to the TSH receptor, thyroglobulin, thyroperoxidase [TPO], and the sodium iodide symporter [NIS]. Another organism, a spirochete called Borrelia burgdorferi has also been implicated.(2-5)
This explains the increased antibodies found on lab panels in Hashimotos patients. These are the antibodies to thyroglobulin and thyroperoxidase (TPO) commonly used by the doctor to confirm a diagnosis of Hashimotos Thyroiditis.
TSH Receptor Antibodies
The TSH Receptor antibodies come in two varieties. In the first type, the TSH receptor is stimulated giving rise to Grave’s Disease and hyperthyroidism. In the second variety, the TSH recepter is blocked giving rise to Hashimotos’s disease. Although both Graves and Hashimotos have a common orign in the molecular mimicry theory, Graves causes a hyperthyroid state, and Hashimotos causes a hypothyroid state. Both very different outcomes. Both Graves and Hashimotos antibodies can coexist in the same patient and one can transform into another.(7)
Gluten and Leaky Gut
How do these Yersenia organisms get into the blood stream to incite an immune response ? The answer is they can “leak in” from the bowel lumen into the blood stream in those patients who suffer from “Leaky Gut”. What causes leaky gut ? The most common cause is gluten consumption in genetically predisposed individuals.
This explains the connection between Hashimotos, Graves and Gluten sensitivity. A gluten free diet is advised for all patient with HAshimotos and Graves disease.
Articles With Related Interest:
Hashimotos Thyroiditis and Selenium Part One
Hashimotos, Selenium and Iodine, Part Two
Hashimotos and Iodine Part Three
Selenium and the Thyroid More Good News
Hashimotos and Molecular Mimicry
Jeffrey Dach MD
7450 Griffin Road Suite 180/190
Davie, Florida 33314
954-792-4663
References and Links
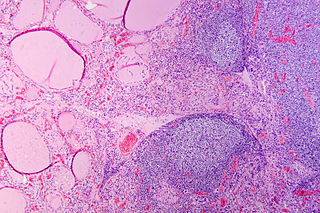
Header image:Hashimoto’s thyroiditis with lymphoid infiltration. Autoantibodies against thyroid peroxidase and thyroglobulin were elevated. Courtesy of WIkimedia Commons.
full text
1) http://www.ncbi.nlm.nih.gov/
J Autoimmun. 2009 May-Jun; 32(3-4): 231–239.
CUTTING EDGE: THE ETIOLOGY OF AUTOIMMUNE THYROID DISEASES by Deirdre Cocks Eschler, Alia Hasham, and Yaron Tomer* Division of Endocrinology, Department of Medicine, Mount Sinai Medical Center One Gustave L. Levy Place, New York, N.Y. 10029,
Molecular Mimicry
2) http://onlinelibrary.wiley.
Prevalence of Yersinia plasmid-encoded outer protein (Yop) class-specific antibodies in patients with Hashimoto’s thyroiditis
S. Chatzipanagiotou1, J. N. Legakis2, F. Boufidou1, V. Petroyianni3, C. Nicolaou1 Clinical Microbiology and Infection
Volume 7, Issue 3, pages 138–143, March 2001
Conclusions There is strong clinical and seroepidemiologic evidence for an immunopathologic causative relationship between Yersinia enterocolitica infection and Hashimoto’s thyroiditis.
3) http://www.ncbi.nlm.nih.gov/
Autoimmun Rev. 2008 Dec;8(2):112-5. Infections and autoimmune thyroid diseases: parallel detection of antibodies against pathogens with proteomic technology. Tozzoli R, Barzilai O, Ram M, Villalta D, Bizzaro N, Sherer Y, Shoenfeld Y.
Different types of infection are implicated in the pathogenesis of autoimmune thyroid diseases (AITD) through molecular mimicry or other mechanisms, but their role is disputed. Human studies support direct or indirect evidence of involvement of some viral and bacterial agents, but reports have provided conflicting and inconclusive results. Using a new automated multiplex array platform for the detection of antibodies, we determined seroreactivity against Toxoplasma gondii, Treponema pallidum, rubella virus, cytomegalovirus, and Epstein-Barr virus in a large group of Italian AITD patients and healthy controls.
Only IgG concentrations against T. gondii were significantly higher in AITD patients than in controls, suggesting that these protozoa may be involved in the initiation of both Hashimoto’s thyroiditis and Graves’ disease.
4) http://www.ncbi.nlm.nih.gov/
Thyroid. 2006 Mar;16(3):225-36.
Human thyroid autoantigens and proteins of Yersinia and Borrelia share amino acid sequence homology that includes binding motifs to HLA-DR molecules and T-cell receptor. Benvenga S, Santarpia L, Trimarchi F, Guarneri F.
We previously reported that the spirochete Borrelia burgdorferi could trigger autoimmune thyroid diseases (AITD). Subsequently, we showed local amino acid sequence homology between all human thyroid autoantigens (human thyrotropin receptor [hTSH-R], human thyroglobulin [hTg], human thyroperoxidase [hTPO], human sodium iodide symporter [hNIS]) and Borrelia proteins (n = 6,606), and between hTSH-R and Yersinia enterocolitica (n = 1,153). We have now updated our search of homology with Borrelia (n = 11,198 proteins) and extended our search on Yersinia to the entire species (n = 40,964 proteins). We also searched the homologous human and microbial sequences for peptide-binding motifs of HLA-DR molecules, because a number of these class II major histocompatibility complex (MHC) molecules (DR3, DR4, DR5, DR8, and DR9) are associated with AITD. Significant homologies were found for only 16 Borrelia proteins (5 with hTSH-R, 2 with hTg, 3 with hTPO, and 6 with hNIS) and only 19 Yersinia proteins (4 with hTSH-R, 2 with hTg, 2 with hTPO, and 11 with hNIS). Noteworthy, segments of thyroid autoantigens homologous to these microbial proteins are known to be autoantigenic. Also, the hTSH-R homologous region of one Borrelia protein (OspA) contains an immunodominant epitope that others have found to be homologous to hLFA-1. This is of interest, as the hLFA-1/ICAM-1 ligand/receptor pair is aberrantly expressed in the follicular cells of thyroids affected by Hashimoto’s thyroiditis. A computer-assisted search detected antigenic peptide binding motifs to the DR molecules implicated in AITD. In conclusion, our in silico data do not directly demonstrate that Borrelia and Yersinia proteins trigger AITD but suggest that a restricted number of them might have the potential to, at least in persons with certain HLA-DR alleles.
5) http://www.ncbi.nlm.nih.gov/
Thyroid. 2004 Nov;14(11):964-6.
Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Benvenga S, Guarneri F, Vaccaro M, Santarpia L, Trimarchi F.
Subclinical exposure to microbic antigens that share amino acid sequence homology with self antigens might trigger autoimmune diseases in genetically predisposed individuals via molecular mimicry. Genetic predisposition to Graves’ disease (GD) or Hashimoto’s thyroiditis (HT) is conferred by HLA loci DR3 or DR5, respectively. Yersinia enterocolitica (YE) outer proteins (YOPs) are candidate triggers based on the high prevalence of serum antibodies (Ab) against YOPs in autoimmune thyroid diseases (AITD) and reactivity of these Ab with hTSH-R, suggesting homology between YOPs and hTSH-R. We have reported previously that the spirochete Borrelia burgdorferi (Bb) could be another trigger. We have explored further the homology of hTSH-R with YE and Bb. Using the Basic Local Alignment Search Tool (BLAST), we found four matches with YE and five matches with Bb . Residues 22-272, 186-330, 319-363 and 684-749 of hTSH-R matched YopM, Ysp, exopolygalacturonase and SpyA of YE (identity 23-31%, similarity 40-48%). Residues 112-205, 127-150, 141-260, 299-383 and 620-697 of hTSH-R matched outer surface protein A, flagellar motor rotation protein A, two hypothetical proteins (BBG02 and BBJ08) and DNA recombinase/ATP dependent helicase of Borrelia (identity 27-50%, similarity 40-75%). Interestingly, the above hTSH-R regions coincide with (or include) known human T-cell epitopes: aa 52-71, 140-176, 240-270, 340-380 and 441-661. Our data strengthen the hypothesis of Bb and YE as environmental triggers of AITD in genetically predisposed persons through molecular mimicry mechanisms.
6) http://www.ncbi.nlm.nih.gov/pubmed/16617190
Intern Med. 2006;45(6):385-9. Four cases of Graves’ disease which developed after painful Hashimoto’s thyroiditis. Ohye H, Nishihara E, Sasaki I, Kubota S, Fukata S, Amino N, Kuma K, Miyauchi A.
We report four cases of Graves’ disease that developed after painful Hashimoto’s thyroiditis. All were middle-aged women, who had high titers of anti-thyroid antibodies and thyrotoxicosis at the onset of painful Hashimoto’s thyroiditis. After 2 to 7 years, they developed Graves’ disease with positive antibody against the thyrotropin receptor. Their clinical courses of Graves’ disease went favorably due to the treatment with antithyroid drug or radioactive iodine therapy. Painful Hashimoto’s thyroiditis is an atypical variant of Hashimoto’s thyroiditis and is one form of destructive thyroiditis. Thyroid damage due to painful Hashimoto’s thyroiditis may be associated with the development of Graves’ disease.
Hashimotos Ataxia Encephalopathy
BMJ
7) http://www.ncbi.nlm.nih.gov/
http://www.ncbi.nlm.nih.gov/
BMJ Case Rep. 2010 Nov 23;2010. A series report of autoimmune hypothyroidism associated with Hashimoto’s encephalopathy: an under diagnosed clinical entity with good prognosis. Nayak HK, Daga MK, Kumar R, Garg SK, Kumar N, Mohanty PK.
Thyroid dysfunctions may be accompanied by numerous neurological and psychiatric disorders. The most well-known is cognitive impairment and depression in hypothyroid patients, as well as an increased risk of cerebrovascular accidents. A separate, although a rare entity, is Hashimoto’s encephalopathy. Unlike encephalopathy associated with other conditions, management in Hashimoto’s encephalopathy highly responds to steroid treatment and may be associated with normal thyroid profile at presentation. Hashimoto’s encephalopathy, while rare, may have been under-recognised since its clinical presentation overlaps several more common disorders, such as depression, seizures or anxiety. We present two cases of hypothyroidism with peculiar presentation. The first case has rapidly progressive neurological dysfunction, normal thyroid function at presentation, normal MRI finding and responds to steroid treatment. The second case has a subacute progressive neurological deterioration with elevated thyroid-stimulating hormone titre at presentation. Both these cases are known hypothyroidism on regular thyroxin replacement therapy with elevated anti-thyroid peroxidase antibodies. We conclude that Hashimoto’s encephalopathy can present with a wide spectrum of neurological illnesses in the setting of hypothyroidism. Thyroid status may vary from hypothyroid, normothyroid to even hyperthyroid. This condition usually has an abnormal electroencephalography (EEG) background and usually responds to high dose steroids.
When should the diagnosis of Hashimoto’s encephalopathy be entertained? Any neuropsychiatric condition that is not responding to conventional treatment, especially in the setting of probable or known autoimmune thyroiditis, should raise suspicion for Hashimoto’s encephalopathy.
8) http://www.ncbi.nlm.nih.gov/
Brain Nerve. 2013 Apr;65(4):365-76.
[Hashimoto’s encephalopathy and autoantibodies].
[Article in Japanese] Yoneda M.
Encephalopathy occasionally occurs in association with thyroid disorders, but most of these are treatable. These encephalopathies include a neuropsychiatric disorder associated with hypothyroidism, called myxedema encephalopathy. Moreover, Hashimoto’s encephalopathy (HE) has been recognized as a new clinical disease based on an autoimmune mechanism associated with Hashimoto’s thyroiditis. Steroid treatment was successfully administered to these patients.
Recently, we discovered that the serum autoantibodies against the NH2-terminal of α-enolase (NAE) are highly specific diagnostic biomarkers for HE.
Further, we analyzed serum anti-NAE autoantibodies and the clinical features in many cases of HE from institutions throughout Japan and other countries. Approximately half of assessed HE patients carry anti-NAE antibodies. The age was widely distributed with 2 peaks (20-30 years and 50-70 years). Most HE patients were in euthyroid states, and all patients had anti-thyroid (TG) antibodies and anti-thyroid peroxidase (TPO) antibodies. Anti-TSH receptor (TSH-R) antibodies were observed in some cases. The common neuropsychiatry features are consciousness disturbance and psychosis, followed by cognitive dysfunction, involuntary movements, seizures, and ataxia. Abnormalities on electroencephalography (EEG) and decreased cerebral blood flow on brain SPECT were common findings, whereas abnormal findings on brain magnetic resonance imaging (MRI) were rare. HE patients have various clinical phenotypes such as the acute encephalopathy form, the chronic psychiatric form, and other particular clinical forms, including limbic encephalitis, progressive cerebellar ataxia, and Creutzfeldt-Jakob disease (CJD)-like form. The cerebellar ataxic form of HE clinically mimics spinocerebellar degeneration (SCD) and is characterized by the absence of nystagmus, absent or mild cerebellar atrophy, and lazy background activities on EEG. Taken together, these data suggest that the possibility of encephalopathy associated with thyroid disorders must be considered.
9) http://www.ncbi.nlm.nih.gov/
J Neurol Neurosurg Psychiatry. 2007 February; 78(2): 196–197.
Hashimoto’s encephalopathy presenting with progressive cerebellar ataxia. H Nakagawa, M Yoneda, A Fujii, K Kinomoto, and M Kuriyama H Nakagawa, M Yoneda, A Fujii, K Kinomoto, M Kuriyama, Second Department of Internal Medicine, Faculty of Medical Sciences, University of Fukui, Fukui, Japan
Recently, we reported serum autoantibodies against the amino (NH2) terminal region of α enolase (NAE) as a useful diagnostic marker of Hashimoto’s encephalopathy
We describe here a patient with Hashimoto’s encephalopathy, who presented with progressive cerebellar ataxia with mild abnormality on electroencephalography (EEG) and showed marked improvement after steroid administration. The patient was diagnosed as having Hashimoto’s encephalopathy owing to the presence of the anti‐NAE antibodies as well as antithyroid antibodies in the serum.
10) http://www.ncbi.nlm.nih.gov/
Rinsho Byori. 2009 Mar;57(3):271-8.
[Anti-NAE autoantibodies and clinical spectrum in Hashimoto’s encephalopathy]. Matsunaga A, Yoneda M.
Hashimoto’s thyroiditis (HT) is the most common disorder affecting the thyroid gland. Encephalopathy associated with abnormal thyroid functions, such as myxedema encephalopathy, is treatable. Hashimoto’s encephalopathy (HE) was recognized as a new clinical disease based on an autoimmune mechanism associated with HT, and steroid treatment has been successfully administrated. Recently, we discovered serum autoantibodies against the NH2-terminal of a-enolase (NAE) as a specific diagnostic marker for HE. We analyzed these serum anti-NAE autoantibodies and the clinical features in 84 cases of HE. The 84 patients consisted of 26 men and 58 women, from many institutions throughout Japan and other countries. A total of 37 patients carried anti-NAE antibodies (44%). The age was widely distributed between 19 and 87 years old, with two peaks (around 20-30 and 50-70 years old). Most patients were in euthyroid states, and all patients had anti-thyroid (TG) and/or anti-thyroid peroxidase (TPO) antibodies, and anti-TSH receptor (TSHR) antibodies in some cases. Only 20% of patients had past histories of HT. The acute encephalopathy form was the most common clinical feature, and subacute or chronic psychiatric forms and other forms such as pure ataxia, limbic encephalopathy, and Creutzfeldt Jakob-like forms followed. The patients with anti NAE antibodies tended to exhibit acute encephalopathy. The most common neuropsychiatric features were consciousness disturbance, psychiatric symptoms, and seizures. Involuntary movements (tremor, myoclonus, or choreoathetosis) or ataxia occasionally occurred. Abnormalities, especially the slowing of background activities, on EEG and elevated levels of protein/IgG in cerebrospinal fluid (CSF) were common and useful laboratory findings for the diagnosis, while abnormalities on brain MRI were rare and non-specific in HE. Immunotherapies such as glucocorticoid, immunosuppressants, immunoglobulin, and plasma exchange, were recommended and effective for HE treatment. HE belongs to a part of a clinical spectrum consisting of individuals with anti-thyroid antibodies, overlapping the clinical spectrum of HT. Anti-NAE autoantibodies were positive in 44% of patients with HE. Considering the overall findings, we should be aware of the possibility of autoimmune encephalopathy associated with thyroid disorders (HE) in patients with an unknown etiology of neuronpsychiatric symptoms with/without a past history of HT.
11) http://www.ncbi.nlm.nih.gov/
J Neurol Neurosurg Psychiatry. 2001 Jul;71(1):81-7.
Ataxia associated with Hashimoto’s disease: progressive non-familial adult onset cerebellar degeneration with autoimmune thyroiditis. Selim M, Drachman DA. University of Massachusetts Medical School, 55 Lake Ave North, Worcester, MA 01655, USA.
12) http://www.ncbi.nlm.nih.gov/
Indian J Pediatr. 2007 May;74(5):492-4.
Hashimoto’s encephalopathy in an adolescent boy.
Ray M, Kothur K, Padhy SK, Saran P.
Hashimoto’s encephalopathy is an under-recognized cause of acute encephalopathy both in children and adults. We hereby describe a 12.5 yr old boy with this rare disorder that presented with an acute onset of episodic psychosis with hallucinations along with seizures and had elevated antithyroid antibodies. Symptoms improved with thyroxine replacement and anticonvulsants and EEG normalized 3 mth into follow up. Hashimoto’s encephalopathy should be considered in patients with unexplained encephalopathy and seizures, as prompt recognition and management can lead to an excellent outcome.
13) http://www.ncbi.nlm.nih.gov/
Pediatr Neurol. 2011 Dec;45(6):420-2.Hashimoto’s encephalopathy in children and adolescents.Erol I, Saygi S, Alehan F. Hashimoto’s encephalopathy is an underdiagnosed, steroid-responsive, progressive or relapsing encephalopathy associated with high titers of serum antithyroid antibodies. Although Hashimoto’s encephalopathy is well documented in adults, it is rarely observed or studied in children and adolescents. We describe the clinical and laboratory findings of four children (aged 9-15 years) with Hashimoto’s encephalopathy. The clinical features of two patients at presentation included epileptic seizures and confusion. The other presenting signs included breath-holding spells, behavioral problems, psychosis, and ataxia (one patient each). During their presentation, three patients were euthyroid, and one was hyperthyroid. All patients manifested increased antithyroid antibodies, and all improved with steroid treatment. Hashimoto’s encephalopathy is rarely suspected at presentation. Therefore, greater awareness of its signs by clinicians is necessary for proper diagnoses.
14) http://www.ncbi.nlm.nih.gov/
East Asian Arch Psychiatry. 2011 Mar;21(1):32-6. Presenile dementia: a case of Hashimoto’s encephalopathy. Chong CS, Leung JL, Wong IH, Ng PW, Miao MY. Hashimoto’s encephalopathy may present with a variety of neurological symptoms and signs, including myoclonus, epileptic seizures, disturbance of consciousness, psychosis, ataxia, and presenile dementia. This report is of a 57-year-old woman with a history of thyroid disease who was investigated for generalised seizures, rapid decline in cognitive function, increasing dependency, and gradual change in personality. High thyroid autoantibody titres confirmed the diagnosis of Hashimoto’s encephalopathy and her symptoms improved with treatment with prednisolone. The differential diagnosis of presenile dementia, aetiology and pathogenesis of Hashimoto’s encephalomyelitis, and treatment options are discussed. Hashimoto’s encephalomyelitis should be considered in the differential diagnosis of presenile dementia, particularly in patients with a history of thyroid disease.
15) http://www.ncbi.nlm.nih.gov/
Rinsho Shinkeigaku. 2002 Feb;42(2):162-6.Hashimoto’s encephalopathy–case report and diagnostic issues in Japan]. [Article in Japanese] Nakamura H, Tokonami F, Yamasaki M.
Epileptic Disord. 2011 Sep;13(3):253-8. Non-convulsive status epilepticus of frontal origin as the first manifestation of Hashimoto’s encephalopathy. Monti G, Pugnaghi M, Ariatti A, Mirandola L, Giovannini G, Scacchetti S, Nichelli P, Meletti S.Hashimoto’s encephalopathy is an often misdiagnosed, life threatening, condition which improves promptly with steroid therapy. Since clinical manifestations are heterogeneous and non-specific, the diagnosis is often difficult. Several case reports of Hashimoto’s encephalopathy presenting with partial or generalised seizures are described, but only a few have focused on status epilepticus as the first clinical manifestation. We report two patients presenting with repetitive and prolonged seizures characterised by progressive reduction in contact and reactivity associated with frontal/diffuse polyspike-and-wave activities. This condition, which can be interpreted as a form of non-convulsive status epilepticus (NCSE) of frontal origin, was refractory to antiepileptic drugs but responded promptly to high doses of intravenous steroid treatment. In cases of unexplained encephalopathy with EEG documentation of NCSE, the early recognition and treatment of Hashimoto’s encephalopathy may lead to a favourable prognosis. [Published with video sequences].
J Clin Neurosci. 2008 Nov;15(11):1301-4. Hashimoto’s encephalopathy masquerading as acute psychosis.
Wilcox RA, To T, Koukourou A, Frasca J.Hashimoto’s encephalopathy (HE) is a relapsing, but exquisitely corticosteroid-responsive encephalopathy associated with autoimmune thyroiditis. Although a rare disease, with just over 100 cases reported, it may be under-recognised. Its presentation can be protean with prominent neuropsychiatric features, stroke-like episodes, seizures and myoclonic jerks. Prompt corticosteroid treatment usually leads to rapid recovery. Here we report a patient with HE, initially presenting with florid neuropsychiatric symptoms. Recent developments in the understanding of this condition are discussed.
Srp Arh Celok Lek. 2005 Oct;133 Suppl 1:88-91.
[Autoimmune thyroid disease and brain]. [Article in Serbian] Zarković M.
Changes of the affective and cognitive function are usually associated with thyroid gland dysfunction. In autoimmune thyroid disease, these changes can be caused by thyroid dysfunction (hypo- or hyperthyroidism) or associated with the presence of antithyroid antibodies. Even a small change in thyroid hormone concentration is associated with change of cognitive function. In euthyroid older males, variation of total and free thyroxin accounts for about 10% of Wechsler adult intelligence test variance. In euthyroid females, lower cognitive function, measured by Mini Mental test, also correlates with blood thyroxin. Short-term (4 weeks) hypothyroidism induces clinically significant cognitive dysfunction, which is reversible by thyroid hormone substitution. Mild hypothyroidism (TSH less than 10) also induces reversible cognitive dysfunction. In hypothyroidism, PET scanning shows global reduction of brain blood flow and glucose metabolism. Hashimoto’s encephalopathy is characterized by corticosteroid reversible encephalopathy associated with the presence of antithyroid antibodies. Encephalopathy can be manifested as multiple stroke-like episodes (vasculitis like), or as diffuse, progressive type characterized by dementia and psychiatric symptoms. In euthyroid patients with Hashimoto’s thyroiditis and no evidence of neurological disease, SPECT showed brain perfusion abnormalities. Post mortem and brain biopsy findings can be normal or show perivascular lymphocytic infiltration. Recently, presence of antineuronal antibodies has been found in patients with Hashimoto’s thyroiditis. Specific high reactivity against human alpha-enolase was high in patients with Hashimoto’s encephalopathy, but absent in patients with other neurological disorders and healthy subjects. Specific antineural antibodies were found in another group of Hashimoto’s encephalopathy patients. Furthermore, Ferracci et al, found antithyroid antibodies in the CSF of patients with Hashimoto’s encephalopathy.
Intern Emerg Med. 2006;1(1):15-23. Clinical and diagnostic aspects of encephalopathy associated with autoimmune thyroid disease (or Hashimoto’s encephalopathy). Tamagno G, Federspil G, Murialdo G.Encephalopathy associated with autoimmune thyroid disease, currently known as Hashimoto’s encephalopathy, but also defined as corticosteroid-responsive encephalopathy associated with autoimmune thyroiditis, is a relatively rare condition observed in a small percentage of patients presenting with autoimmune thyroid disease. It consists of a subacute, relapsing-remitting, steroid-responsive encephalopathy characterised by protean neurologic and neuropsychiatric symptoms, diffuse electroencephalographic abnormalities and increased titres of antithyroid antibodies in serum and/or in cerebrospinal fluid. Most of the cases presenting this neurologic complication are affected by Hashimoto’s thyroiditis or, less frequently, by other autoimmune thyroid diseases, chiefly Graves’ disease. The pathogenesis of this encephalopathy is still unknown and largely debated, because of extremely varied clinical presentation, possibly referable to different aetiologic and pathophysiologic mechanisms, as confirmed by the two clinical cases we report in this paper. Autoimmune aetiology is, however, very likely in view of the well established favourable response to corticosteroid administration. Both vasculitis and autoimmunity directed against common brain-thyroid antigens represent the most probable aetiologic pathways. Clinical manifestations include consciousness changes, neurologic diffuse or focal signs, headache, and altered cognitive function. Although unspecific, cerebral oedema has also been described. Cerebrospinal fluid examination often discloses an inflammatory process, with a mild increase in protein content and occasionally in lymphocyte count. In this review, clinical criteria for the diagnosis of defined, probable, or possible encephalopathy associated with autoimmune thyroid disease are suggested. Corticosteroid therapy currently allows us to obtain rapid remission of disease symptoms, but adverse outcomes as well as spontaneous remissions have also been reported.21) http://www.ncbi.nlm.nih.gov/
Best Pract Res Clin Endocrinol Metab. 2005 Mar;19(1):53-66.
Hashimoto’s encephalopathy: myth or reality? An endocrinologist’s perspective. Fatourechi V.Since the first description of a case of episodic encephalopathy associated with Hashimoto’s thyroiditis in 1966, many cases of corticosteroid-responsive encephalopathy associated with positive antithyroid antibodies, clinical Hashimoto’s thyroiditis, or spontaneous autoimmune thyroid failure have been reported. These patients have neurologic manifestations of encephalopathy unrelated to other known causes. The condition has thus been termed ‘Hashimoto’s encephalopathy’. The literature shows no proven association between thyroid disease and the neurologic process. Although the association of a common endocrinologic condition and a rare neurologic disease may occur by chance, this type of encephalopathy probably has an autoimmune nature and thus is more likely to occur in the background of another autoimmune condition such as autoimmune thyroid disease. Until the pathogenesis of these coincident conditions is better defined, the term ‘corticosteroid-responsive encephalopathy associated with autoimmune thyroiditis’ is more accurate and descriptive than Hashimoto’s encephalopathy. Advances in the field may clarify this seemingly inconsistent terminology.
——————————
Gluten Ataxia
22) http://www.ncbi.nlm.nih.gov/
Neurology. 2006 Feb 14;66(3):373-7. Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Hadjivassiliou M, Mäki M, Sanders DS, Williamson CA, Grünewald RA, Woodroofe NM, Korponay-Szabó IR.
To investigate the presence of autoantibody deposition against type 2 tissue transglutaminase (TG2; a reliable marker of the whole spectrum of gluten sensitivity) in the jejunal tissue and brain of patients with gluten ataxia and in control subjects.
METHODS:
The authors evaluated jejunal biopsy samples from nine patients with gluten ataxia and seven patients with other causes of ataxia for the presence of TG2-related immunoglobulin deposits using double-color immunofluorescence. Autopsy brain tissue from one patient with gluten ataxia and one neurologically intact brain were also studied.
RESULTS:
IgA deposition on jejunal TG2 was found in the jejunal tissue of all patients with gluten ataxia and in none of the controls. The intestinal IgA deposition pattern was similar to that seen in patients with overt and latent celiac disease and in those with dermatitis herpetiformis. Widespread IgA deposition around vessels was found in the brain of the patient with gluten ataxia but not the control brain. The deposition was most pronounced in the cerebellum, pons, and medulla.
CONCLUSIONS:
Anti-tissue transglutaminase IgA antibodies are present in the gut and brain of patients with gluten ataxia with or without an enteropathy in a similar fashion to patients with celiac disease, latent celiac disease, and dermatitis herpetiformis but not in ataxia control subjects. This finding strengthens the contention that gluten ataxia is immune mediated and belongs to the same spectrum of gluten sensitivity as celiac disease and dermatitis herpetiformis.
23) http://www.ncbi.nlm.nih.gov/
Ann Neurol. 2008 Sep;64(3):332-43. doi: 10.1002/ana.21450.
Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D.
Gluten sensitivity typically presents as celiac disease, a chronic, autoimmune-mediated, small-intestinal disorder. Neurological disorders occur with a frequency of up to 10% in these patients. However, neurological dysfunction can also be the sole presenting feature of gluten sensitivity. Development of autoimmunity directed toward different members of the transglutaminase gene family could offer an explanation for the diversity in manifestations of gluten sensitivity. We have identified a novel neuronal transglutaminase isozyme and investigated whether this enzyme is the target of the immune response in patients with neurological dysfunction.
METHODS:
Using recombinant human transglutaminases, we developed enzyme-linked immunosorbent assays and inhibition assays to analyze serum samples of patients with gluten-sensitive gastrointestinal and neurological disorders, and various control groups including unrelated inherited or immune conditions for the presence and specificity of autoantibodies.
RESULTS:
Whereas the development of anti-transglutaminase 2 IgA is linked with gastrointestinal disease, an anti-transglutaminase 6 IgG and IgA response is prevalent in gluten ataxia, independent of intestinal involvement. Such antibodies are absent in ataxia of defined genetic origin or in healthy individuals. Inhibition studies showed that in those patients with ataxia and enteropathy, separate antibody populations react with the two different transglutaminase isozymes. Furthermore, postmortem analysis of brain tissue showed cerebellar IgA deposits that contained transglutaminase 6.
INTERPRETATION:
Antibodies against transglutaminase 6 can serve as a marker in addition to human leukocyte antigen type and detection of anti-gliadin and anti-transglutaminase 2 antibodies to identify a subgroup of patients with gluten sensitivity who may be at risk for development of neurological disease.
24) http://www.plosone.org/
Anti Transglutaminase Antibodies Cause Ataxia in Mice
Sabrina Boscolo Marios Hadjivassiliou, March 15, 2010
Gluten sensitive enteropathy or celiac disease (CD) is a common (1 in 90) state of heightened immunological responsiveness to the ingestion of gluten in genetically predisposed individuals having the HLA class II haplotype DQ2 or DQ8 [1]. The clinical spectrum of CD ranges from asymptomatic to the classic malabsorption syndrome [2]. In addition, a variety of neurological manifestations have been identified, including ataxia [3], epilepsy [4], brain atrophy, headache with white matter lesions and polyneuropathy [5].
We found that i) 75% of the CD patient population (without evidence of neurological involvement) has circulating anti-neural IgA and/or IgG antibodies; ii) anti-neural reactivity of serum IgA reflects the presence of anti-TG2 antibodies (belonging to class2), that also cross-react with TG3 and TG6; iii) anti-neural reactivity of serum IgG appears to be determined only in part by the presence of anti-TG2 antibodies; and interestingly, that iv) both TG2-specific (class1) as well as anti-TG2 antibodies crossreacting between TG isozymes (class2) cause ataxia in mice when directly administered to the CNS. This indicates that potentially pathogenic antibodies targeting antigens in the CNS are a common feature among CD patients but that their presence alone is unlikely to be the only event that precipitates into a pathological state.
25) http://link.springer.com/
Sapone, Anna, et al. “Spectrum of gluten-related disorders: consensus on new nomenclature and classification.” (2012).
BMC Medicine February 2012, 10:13,
______________________________
26) http://www.pathologystudent.
PAthology Student: We’ve been discussing thyroiditis lately (see posts from 4/27/09 and 4/28/09). There are four kinds of thyroiditis: Hashimoto, subacute granulomatous, lymphocytic, and fibrosing. The most common of these, by far, is Hashimoto thyroiditis.
Hashimoto is an autoimmune disease in which the patient’s own immune system attacks and slowly destroys the thyroid gland. It’s much more common in women (as is typical of autoimmune diseases), and it is the most common cause of hypothyroidism in parts of the world where there is enough iodine. It typically presents with an enlarged, non-tender thyroid gland. Patients gradually lose thyroid function and eventually become hypothyroid.
27) http://www.pathologyoutlines.
Thyroid gland. Thyroiditis. Hashimoto’s thyroiditis
——————————
28) http://www.ncbi.nlm.nih.gov/
Thyroid. 2005 Jul;15(7):725-9.
Transient Graves’ hyperthyroidism during pregnancy in a patient with Hashimoto’s hypothyroidism. Lu R, Burman KD, Jonklaas J.
The course and severity of autoimmune thyroid disease are altered during pregnancy and in the postpartum period. The thyroidal response to a fluctuating immune status, combined with changes in thyroid economy during pregnancy, may result in a need to adjust the treatment regimen for thyroid disease during pregnancy. Patients with Hashimoto’s hypothyroidism on thyroid hormone replacement are frequently observed to have an increased requirement for levothyroxine early in pregnancy, although this is not a universal finding. Hashimoto’s hypothyroidism does not typically remit during pregnancy, although further progression of thyroiditis may be seen in the postpartum period. Graves’ disease usually improves during pregnancy and flares after delivery, again necessitating monitoring of thyroid status and possible adjustments in thionamide therapy. However, spontaneous transformation from Hashimoto’s hypothyroidism to Graves’ disease during pregnancy is rare. We report a case of transient Graves’ hyperthyroidism during the late second trimester in a patient on levothyroxine replacement for Hashimoto’s hypothyroidism. This resulted in a need to discontinue the patient’s thyroid hormone entirely to avoid exacerbation of her hyperthyroidism. This interesting case is presented, along with a discussion of how the expression of autoimmune thyroid disease may be altered during pregnancy.
29) http://www.ncbi.nlm.nih.gov/
Acta Med Indones. 2010 Jan;42(1):31-5.
Hashimoto’s thyroiditis following Graves’ disease.
Umar H, Muallima N, Adam JM, Sanusi H.
Both Graves’ disease and chronic thyroiditis (Hashimoto’s thyroiditis) are autoimmune diseases of thyroid gland. Graves’ disease is caused by stimulation of TSH receptor located on the thyroid gland by an antibody, which is known as TSH receptor antibody (TRAb). Furthermore, this may lead to hyperplasia and hyperfunction of the thyroid gland. On the contrary, the cause of Hashimoto’s thyroiditis is thought due to a TSH stimulation-blocking antibody (TSBAb) which blocks the action of TSH hormone and subsequently brings damage and atrophy to thyroid gland. Approximately 15-20% of patients with Graves’ disease had been reported to have spontaneous hypothyroidism resulting from the chronic thyroiditis (Hashimoto’s disease). Pathogenesis for chronic thyroiditis following anti-thyroid drug treatment in patients with Graves’ disease remains unclear. It has been estimated that chronic thyroiditis or Hashimoto’s disease, which occurs following the Graves’ disease episode is due to extended immune response in Graves’ disease. It includes the immune response to endogenous thyroid antigens, i.e. thyroid peroxidase and thyroglobulin, which may enhance lymphocyte infiltration and finally causes Hashimoto’s thyroiditis. We report four cases of chronic thyroiditis (Hashimoto’s disease) in patients who have been previously diagnosed with Graves’ hyperthyroidism. In three cases, Hashimoto’s thyroiditis occurs in 7 to 25 years after the treatment of Grave’s disease; while the other case has it only after few months of Grave’s disease treatment. The diagnosis of Hashimoto’s disease (chronic thyroiditis) was based on clinical manifestation, high TSHs level, positive thyroid peroxidase antibody and thyroglobulin antibody, and supported by positive results of fine needle aspiration biopsy. Moreover, the result of histopathological test has also confirmed the diagnosis in two cases. All cases have been successfully treated by levothyroxine treatment.
—————-
pathology pathophysiology
Excellent review article
30) http://www.ncbi.nlm.nih.gov/pubmed/23624127 (free full text)
Hormones (Athens). 2013 Jan-Mar;12(1):12-8.Hashimoto’s Thyroiditis: History and Future Outlook by Yuji Hiromatsu,1 Hiroshi Satoh,2 Nobuyuki Amino3 1Division of Endocrinology and Metabolism, Department of Medicine, Kurume University School of Medicine, Kurume, 2Division of Surgery, Seishinkai Inoue Hospital, Alumni of the 1st Department of Surgery, Kyushu University and
Japanese Society for the Medical History, Itoshima, Fukuoka, 3Kuma Hospital, Center for Excellence in Thyroid Care,
Chuo-ku, Kobe, Japan
Excellent review article-full text
31) http://www.thyroidmanager.org/
Hashimoto’s Thyroiditis January 1, 2012 Takashi Akamizu, M.D., Ph.D. Professor and Chairman, The First Department of Medicine, Wakayama Medical University, 811-1 Kimi-idera, Wakayama 641-8509, Japan, Nobuyuki Amino, M.D. Kuma Hospital, Center for Excellence in Thyroid Care, 8-2-35 Shimoyamate-dori, Chuo-ku, Kobe 650-0011, Japan, Leslie J. DeGroot, M.D.
Research Professor, CELS, University of Rhode Island, 80 Washington St, Providence, RI 02903
Figure 1. Dr. Hakaru Hashimoto
Figure 2. Electron microscopy image of thyroid tissue from a patient with Hashimoto’s thyroiditis, showing electron dense deposits of IgG and TG along the basement membrane of follicular cells.
TSH Blocking Antibodies
An alternative cause of “atrophic” hypothyroidism is the development of thyroid stimulation blocking antibodies (TSBAb), which, as the name implies, prevent TSH binding to TSH-R, but do not stimulate thyroid cells, rather they produce hypothyroidism. It has been proposed that TSBAb bind to epitopes near the carboxyl end of the TSH-R extracellular domain, in contrast to thyroid stimulating antibodies (TSAb), which bind to epitopes near aa 40 at the amino terminus(20). This syndrome occurs in neonates, children and adults. The prevalence of TSBAb in adult hypothyroid patients has been reported to be 10%(21).
However, in contrast to the usual progressive and irreversible thyroid damage occurring in the usual setting, these blocking antibodies tend to follow the course of TSAb–that is, they decrease or disappear over time, and the patient may become euthyroid again(22).
A change from a predominant TSAb response to a predominant TSBAb response can cause patients to have sequential episodes of hyper- and hypothyroid function(23). HLA antigens of hypothyroid patients with TSBAb were found to be different from patients with idiopathic myxedema or Hashimoto’s thyroiditis, and rather similar to patients with Graves’ disease(24).
Anti-NIS antibodies
Antibodies against NIS were found in autoimmune thyroid disease(83). This antibody has an inhibitory activity on iodide transport and may modulate the thyroid function in Hashimoto’s thyroiditis. More recent studies reported rather low prevalence (less than 10%) of anti-NIS antibodies in Hashimoto’s disease and clinical relevance is still unknown(84),(85).
32) http://www.ncbi.nlm.nih.gov/pubmed/7041513
Acta Pathol Jpn. 1982 Jan;32(1):41-56.
Immunohistochemical and electron microscopic studies on Hashimoto’s thyroiditis.Matsuta M.Abstract
The thyroid glands of nine patients with Hashimoto’s thyroiditis were studied by immunoperoxidase method for immunoglobulin (Ig) and thyroid hormone, and of these four tissue specimens were further examined by electron microscope. Immunoperoxidase method for Ig revealed that about 63% of the infiltrating cells contained Ig, and that about 90% of such Ig-containing cells had IgG. IgG-containing cells seemed to secrete autoantibodies. Immunoperoxidase method for thyroid hormone and electron microscopy revealed that there was a good correlation between the morphological features of the thyroid follicle and its immunohistochemical staining pattern. In follicles composed of columnar cells, the colloid and cytoplasm of some epithelial cells were immunohistochemically stained. Dense deposits, regarded as immune complexes, were observed in the basement membrane of these follicles, and lymphocytes were seen between the adjacent cells. When similar deposits appeared in the basement membrane of such follicles, 4 or more lymphocytes per follicle could be seen among the epithelial cells. The present findings seem to indicate that antibody-dependent cell-mediated cytotoxicity plays an important role in the pathogenesis of Hashimoto’s thyroiditis.
33) http://www.ncbi.nlm.nih.gov/
Hum Pathol. 1981 Jun;12(6):561-73.
Ultrastructural pathology in Hashimoto’s thyroiditis.
Shamsuddin AK, Lane RA.
Ultrastructural studies of the thyroid tissue in two cases of Hashimoto’s thyroiditis demonstrated that the inflammatory cells do not pass through the follicular cells. Indeed these cells travelled between the epithelial cells in a manner similar to the neutrophil emigration (diapedesis) through the vessel wall in acute inflammation. Inflammatory infiltrates were composed of lymphocytes, plasma cells, or transformed lymphocytes that showed features intermediate between those of lymphocytes and plasma cells. These inflammatory cells were observed to travel from the stroma to the follicular lumen in a vectorial manner – similar to neutrophilic chemotaxis in acute inflammation. The basement membrane around the thyroid follicles remained intact around some follicles whereas it was reduplicated or focally increased in thickness around others. The basement membrane material seemed to have been secreted by the follicular cells, and strands of early collagen fiber formation were seen within the excess basement membrane material. The follicular cells showed evidence fo sublethal injury characterized by prominent defects of the rough endoplasmic reticulum or the mitochondria. Cells from areas that appeared as foci of squamous metaplasia by light microscopy showed an increased number of cytoplasmic filaments (120 to 160 A), bundles of tonofilaments, large desmosomes, an increased number of desmosomes, and intracellular desmosomes. The colloid content of the follicles was diminished, and it seemed that instead of secreting the protein colloid, the follicular cells in Hashimoto’s thyroiditis were producing either excessive proteinaceous material similar to colloid or other types of proteins such as basement membrane material or keratin.
Jeffrey Dach MD
7450 Griffin Road Suite 180/190
Davie, Florida 33314
954-792-4663
http://www.jeffreydach.com/
http://www.drdach.com/
http://www.naturalmedicine101.com/
http://www.truemedmd.com/
Disclaimer click here: http://www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article:http://wp.me/p3gFbV-1bH
Copyright (c) 2014 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.









Selenium and Thyroid More Good News - Jeffrey Dach MD February 1, 2014 at 10:16 AM
[…] Hashimotos and Molecular Mimicry […]
Iodine and Hashimoto's Autoimmune Thyroid Disease - Jeffrey Dach MD February 7, 2014 at 5:58 PM
[…] Hashimotos, Thyroid Disease and Molecular Mimicry […]
Hashimotos Thyroiditis, Manic Depression, Psychosis and Psychiatric Manifestations - Jeffrey Dach MD February 9, 2014 at 12:19 PM
[…] Hashimotos Thyroid Disease and Molecular Mimicry […]
Celiac Disease and Hashimoto's - Hashimotos Healing January 8, 2015 at 7:11 PM
[…] http://jeffreydachmd.webomg.net/2014/01/hashimotos-thyroid-disease-molecular-mimicry/ […]