 Nicholas Gonzalez MD and the Trophoblastic Theory of Cancer
Nicholas Gonzalez MD and the Trophoblastic Theory of Cancer
By Jeffrey Dach MD
Cancer treatment with chemotherapy yields disappointing results for most cancer cell types. Perhaps we should be exploring alternative cancer treatments, such as one proposed by Nicholas Gonzalez MD, a keynote speaker at a medical conference I attended five years ago in Denver Colorado (Boulderfest conference July 17-20 2008.)
Nicholas Gonzalez MD
Nicholas Gonzalez MD is actively engaged in medical practice in Manhattan where he treats advanced cancer successfully with high dose oral pancreatic enzymes. This treatment regimen is based on the trophoblastic theory of cancer originally proposed by Scottish embryologist John Beard (1858-1924), and resurrected by William Donald Kelley, DDS (1926-2005).
Above Image: Denver Airport courtesy of wikimedia commons.
John Beard and the Trophoblast
John Beard (1858-1924), was a Scottish embryologist who used the light microscope to study developmental embryology as well as cancer pathology. In 1905, Beard was the first to report that trophoblast cells act and behave in a manner identical to cancer cells, acting invasively, and inducing their own blood supply.
What are Trophoblasts?
In the pregnant mammal, trophoblasts are the infiltrative components of the developing embryo which forms the placenta. This invasive, infiltrative behavior is very similar to the way cancer cells infiltrate and invade surrounding tissues. These trophoblast cells are known to produce human chorionic gonadotropin (HCG). In fact, production of HCG is the basis for the widely used pregnancy test. If cancer cells and trophoblast cells are similar, one would expect cancer cells to also produce HCG. That is exactly what they do. This was reported in 1995 by Hernan Acevedo, PhD, and published in Cancer. He found that every cancer produces HCG, same as the trophoblast cells of pregnancy.
Since John Beard’s time 100 years ago, modern molecular biologists have found even more similarities between trophoblasts and malignant cancer cells. Dr. C. Ferretti in the October 2006 issue of Human Reproduction Update, states both cancer and trophoblast cells share the same molecular circuitry for their proliferative, invasive and migratory capacities.
CT Antigens Discovered
Another twist to the story is the recent discovery of a new class of human tumor antigens called CT (cancer/testis) antigens. About 90 genes have been found having messenger RNA expression in both germ cells (testis) and cancer cells, and no expression in otherwise normal cells. This is further evidence linking the trophoblast cells, which are in fact germ cells (also called stem cells), with cancer.
Recent advances in our understanding of molecular biology have shown that John Beard was quite correct to point out the similarity between placental trophoblast cells and malignant cancer cells. Beard’s forgotten predictions in the early 1900’s seem to have an uncanny way of resurfacing 100 years later.
A Diagram Showing the Trophoblast (labeled tr)
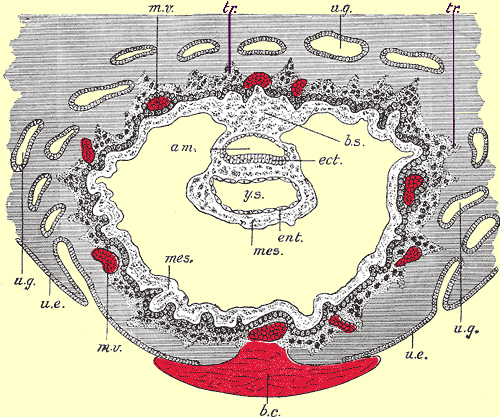 The trophoblast cells in the developing ovum are shown in the diagram below. The trophoblast tissue is labeled tr. Notice the finger-like extensions of the trophoblast cells which invade the endometrium to form the placenta. The layer of trophoblasts induces the maternal blood vessels (shown in red).Section through ovum embedded in the uterine decidua. Semidiagrammatic. Above Image courtesy of wikimedia commons.
The trophoblast cells in the developing ovum are shown in the diagram below. The trophoblast tissue is labeled tr. Notice the finger-like extensions of the trophoblast cells which invade the endometrium to form the placenta. The layer of trophoblasts induces the maternal blood vessels (shown in red).Section through ovum embedded in the uterine decidua. Semidiagrammatic. Above Image courtesy of wikimedia commons.
Legend for the above diagram: am. Amniotic cavity. b.c. Blood-clot. b.s. Body-stalk. ect. Embryonic ectoderm. ent. Entoderm. mes. Mesoderm. m.v. Maternal vessels. tr. Trophoblast. u.e. Uterine epithelium. u.g. Uterine glands. y.s. Yolk-sac.
Pancreatic Enzymes on Day 56
On day 56 of gestation, John Beard observed that the trophoblast cells transform from a malignant, invasive cell type into a mature well behaved cell type. This day 56 also coincides with the appearance of enzyme granules (zymogen granules) in the fetal pancreas. Obviously, since all nutrition comes from the maternal blood supply, the developing fetus has no need for pancreatic enzymes which are needed to digest food consumed after birth. Beard theorized there must be an alternative function for the pancreatic enzymes. He theorized that the appearance of pancreatic enzymes was no accident, and that the most likely explanation was that they were responsible for the transformation of the trophoblast cells behavior from “malignant” to a “benign”, thereby suggesting the use of digestive enzymes to control cancer cells.
Selective Activity of Enzymes on Cancer Cells
The selective activity of trypsin on cancer cells could be explained by the selective digestion of proteins based on isomer structure. Cancer proteins have a right hand isomer structure, and proteins in normal tissue with left hand isomers.(link) However, it is widely known that in cases of acute pancreatitis with release of pancreatic enzymes into the retroperitoneal space, there is obvious autodigestion of human tissue.
A number of studies in both animal models and humans have actually confirmed the utility of pancreatic enzyme for cancer treatment. (link) An excellent review of recent research confirming John Beard’s work as well as the value of enzyme treatments for cancer can be found here. A Critique of the Kelley Nutritional-Metabolic Cancer Program by Melina A. Roberts BSc. From the Townsend Letter for Doctors & Patients June 2003
About the same time as John Beard’s early work, Madam Curie’s (1867-1934) work treating cancer with radiation took the spotlight, and captured the imagination of the media and the public. John Beard’s work on the trophoblast theory was dismissed by mainstream medical science and almost forgotton.
However, the leading biochemist Ernst Krebs wrote this very important 1950 paper supporting the trophoblast theory of cancer:
THE UNITARIAN OR TROPHOBLASTIC THESIS OF CANCER http://www.cancergnosis.com/History/Trophablastic%20theory.pdf
by Ernst T. Krebs, Jr., Ernst T. Krebs, Sr., and Howard H. Beard
(Reprinted From the Medical Record, 163:149-174, July 1950)
William Donald Kelley Resurrects John Beard’s Work
Years later in the 1960’s, William Kelly discovered Beard’s forgotten papers, and resurrected the treatment of cancer with pancreatic enzymes. Kelly had considerable success treating patients with this alternative cancer treatment approach. However, Kelly was a dentist, and bitterly opposed by mainstream medicine, and as expected, had difficulties with the authorities. Kelly was convicted of practicing medicine without a license in 1970, his dental license was suspended in 1976, and he died on Jan. 30, 2005 at the age of 79.
Nicholas Gonzalez’ Research
In 1981, during the Kelly’s early years, a medical student at Cornell Medical School by the name of Nicholas Gonzalez was given a summer project to interview William Donald Kelly and evaluate his results with cancer patients using the pancreatic enzyme treatment. Gonzalez did a retrospective review of 1300 patients who had been treated over a 20-year period with the Kelley protocol with enzymes, diet and nutritional support.
 Gonzalez was so impressed with the data, and the superior patient outcomes, that this summer project expanded into a book, and later adopted as his own life’s work.
Gonzalez was so impressed with the data, and the superior patient outcomes, that this summer project expanded into a book, and later adopted as his own life’s work.
Left Image: William Kelly courtesy of Peter Barry Chowka
Continuing after Kelly, Nicholas Gonzalez MD carried on with his legacy at a Manhattan office, documenting remarkable success over the past decade or so. Selected case reports from the Gonzalez Manhattan office show dramatic clinical results not possible with conventional mainstream cancer treatment. This information is posted on the Dr Gonzalez web site.
In 1999, Gonzalez published a 2 year pilot study of 10 patients with inoperable advanced pancreatic cancer treated with large doses of orally ingested pancreatic enzymes. Results showed 80% survival after 1 year, 45% survival after 2 years and 36% survival after 3 years. (Gonzalez and Isaacs, 1999). These results are far above the 25% one year, 10% two year, and 6 % three year survival reported in the National Cancer Data Base for inoperable pancreatic cancer (Niederhuber, Brennan and Menck, 1995).
Shortly after this, Gonzalez received a $1.4 million grant from the National Center for Complementary and Alternative Medicine at the National Institutes of Health for further study on enzyme therapy and pancreatic cancer. The study was conducted at Columbia-Presbyterian Medical Center in New York under the supervision of the NCI and with approval from the FDA. The outcome of this study has not yet been published.
Listen to Nicholas Gonzalez MD lecture exerpt on Beard and the Trophoblastic Theory of Cancer and Treatment with Pancreatic Enzymes. Click here to listen to recording: http://www.newspringpress.com/1105speech.mp3
Otto Warburg
Otto Warburg made important contributions to the understanding of the metabolic activity of cancer. All cancers, as well as trophoblast cells have a high glucose utilization using the primitive glycolysis pathway. They tend not to use oxidative phosphorylation and thrive in a low oxygen environment. This is known as the Warburg Effect. Recent work has built on Warburg’s ideas using inhibitors of glycolysis such as 3 bromopyruvate to kill cancer cells by stopping glycolysis.
Here are two articles describing inhibition of cancer cell glyocolysis with 3 bromo pyruvate:
http://cancerres.aacrjournals.org/cgi/content/full/62/14/3909
fulll text “Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production”. Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL (2002).
Cancer Res. 62 (14): 3909–13. PMID 12124317.
http://www.kosen21.org/upload_repository2/community/01230411451209597a.pdf
full text REVIEW Glycolysis inhibition for anticancer treatment
H Pelicano1, DS Martin2,{, R-H Xu3 and P Huang1 Oncogene (2006) 25, 4633–4646
Others have used insulin induced hypoglycemia to starve cancer cells of their glucose substrate. Avid glucose uptake is the basis of modern PET (positron emission tomography) imaging of the body which is capable of showing cancer deposits with radiolabeled 17-Flouro-deoxyglucose.
Aneuploidy – and Confined Placental Mosaicism
Recently there has been resurgence of interest in aneuploidy and cancer championed by Peter Duesberg in Chromosomal Chaos and Cancer. [P. Duesberg (2007) Scientific American 296, May 52-59]. Aneuploidy or abnormal and multiple sets of chomosomes is commonly found in cancer, and also in the placenta. Using ultrasound guided chorionic villous sampling, it has been discovered that between 2-5% of placental samples show aneuploid cells; this is called confined placental mosaicism, which apparently does not affect the developing embryo.
No Coexistence of Cancer with Circulating Enzymes of Pancreatitis
One last point I am compelled to mention. During my 30 year career as a radiologist much of my time was spent reading images of metastatic cancer on CAT scans. One of the things I noticed was that I never witnessed the presence of metastatic cancer in patients who had pancreatic enzymes circulating freely in the bloodstream from acute or chronic diffuse pancreatitis. Excluded of course was focal pancreatitis caused by an obstructed pancreatic duct due to a small pancreatic cancer. Thus I had independently confirmed the major tenet of John Beard and Ernst Krebs many years before I even heard of the trophoblastic theory of cancer.
Cancer of Small Bowel Relatively Rare
Another observation most experienced radiologists and surgeons will make is the relative rarity of neoplasm involving the small bowel compared to the relative common appearance (50 times more common) of neoplasm in the colon and the stomach. Ernst Krebs makes this same observation in his landmark 1950 paper on the unified trophoblast theory of cancer, and Krebs suggests that pancreatic enzymes released into the duodenum at the duct of Wirsung and Santorinin are responsible for this 50 times reduction in small bowel cancer.
see: THE UNITARIAN OR TROPHOBLASTIC THESIS OF CANCER http://www.cancergnosis.com/History/Trophablastic%20theory.pdf
by Ernst T. Krebs, Jr., Ernst T. Krebs, Sr., and Howard H. Beard
(Reprinted From the Medical Record, 163:149-174, July 1950)
The age-adjusted death rate for cancer of the colon is 47 times higher than cancer of the small bowel, at 0.4 for small bowel and 18.8 for colon cancer per 100,000 men and women per year. (NIH NCI SEER web site http://seer.cancer.gov/statfacts/
NIH Grant Proposal to Study Cancer
The NIH (National Institute of Health) has spent literally trillions over four decades on failed cancer research. It is time to take a different approach with a few proposals to investigate the trophoblast theory of cancer.
A widely used technique in molecular biology is the tracer study. The older tracer method involved the use of Carbon 14 radio-labeling. The newer method uses insertion of the green florescent protein (GFP) into the protein one wishes to study.
Carbon 14 Radio-Labeled Trypsin
The proposed study can be done by using Carbon 14 radio-labeling of key amino acids in the pancreatic enzyme, trypsin, and feeding these radio-labeled amino acids to the pigs used to harvest the trypsin for later use. Then administer the radio-labeled trypsin enzymes to an animal model of cancer looking for the distribution of the radio-label in the sacrificed animals. If there is an effect on the cancer cells, I would expect to find the radio-labeled enzymes at the surface of the cancer cells.
GFP Green Florescent Protein
Another more elegant approach would be to genetically modify the pancreatic trypsin enzyme in mice by adding a green florescent marker gene (GFP), a common technique used in molecular biology. If pancreatic enzymes control the trophoblast, then the experiments should confirm the presence of the florescent marker at the trophoblast cells after day 56 in the developing embryo.
To study cancer, the green florescent gene (GFP) can be inserted into DNA of the animals (usually pigs) used to manufacture the pancreatic enzymes. These labeled enzymes which can then be administered to mice pretreated with cancer cells. The survival of the treated vs. control mice as well as the fate of the labeled enzymes would be useful to know about. If the enzymes are having an effect on the cancer cells, then I would expect the florescent label or radio-label to be found at the tumor site.
Using the NIH to Find a Cure for Cancer
A few decades ago, Richard Nixon, declared a war against cancer and ramped up funding for NIH research which mostly went towards proving the idea that cancer was caused by a virus. This line of research expended massive amounts of money and ended a dismal failure. A new and more promising direction for cancer research would be to investigate the mysteries of the trophoblast which shares so many features in common with cancer cells. We now have the molecular tools that John Beard a century ago could only imagine. How do we get the NIH to pursue this? Use political pressure by contacting your congressman and asking them to push the NIH to fund the research.
Conclusion:
Advances in molecular biology now make it fairly straightforward to validate and expand on the early work of Scottish embryologist John Beard, Ernst Krebs and Otto Warburg. The research costs for such a program would be minimal and the potential gains enormous.
Update 2014: Excellent article by Dr Gonzales on the Enzyme Treatment of cancer: History_Enzyme_Treatment_Cancer_Nicholas_Gonzalez_2014
Articles with Related Interest : Cancer as a Metabolic Disease
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Florida 33314
954-792-4663
http://www.drdach.com/
http://www.naturalmedicine101.com/
http://www.truemedmd.com/
http://www.bioidenticalhormones101.com/
References and Links
http://www.dr-gonzalez.com/index.htm
Nicholas J. Gonzalez, M.D. Linda L. Isaacs, M.D.
36A East 36th Street, Suite 204
New York, N.Y. 10016
Phone: 212-213-3337 Fax: 212-213-3414
http://www.michaelspecter.com/ny/2001/2001_02_05_gonzalez.html
annals of medicine the outlaw doctor Cancer researchers used to call him a fraud. What’s changed? february 5, 2001 Michael Specter The New Yorker
http://www.dr-gonzalez.com/totalhealth_7b_00.htm
most of his talk from the meeting on John Beard’s theories
http://www.alternative-therapies.com/at/web_pdfs/isaacs.pdf
EVALUATING ANECDOTES AND CASE REPORTS Linda L. Isaacs, MD
http://www.prevention.com/cda/article/alternative-medicine-saved-our-lives/4b8b9f9ad6914110VgnVCM10000013281eac____/health/healthy.living.centers/cancer/
Alternative Medicine Saved Our Lives How unconventional treatments paid off for 4 desperately ill womenTalk to these four women and their health care providers on our discussion forums September 4 through 30.
http://www.alternative-therapies.com/at/web_pdfs/gonzalez1.pdf
THE GONZALEZ THERAPY AND CANCER:A COLLECTION OF CASE REPORTS
Nicholas J. Gonzalez, MD; Linda L. Isaacs, MD
http://www.dr-gonzalez.com/best_cases.htm
On July 7, 1993, at the NCI I presented 25 histories of my “best cases”: patients with diagnosed, biopsy-proven cancer who enjoyed either documented regression of disease or long-term survival on their nutritional protocol. Here are three of those 25 cases:
I. F., a 68-year-old woman, underwent a left mastectomy for carcinoma in July 1987; 1 of 7 lymph nodes was found positive. She was initially treated with tamoxifen; but when a CAT scan in September 1988 showed metastatic disease in both lobes of the liver, she was started on CMF chemotherapy (Cytoxan, methotrexate, and 5-fluoracil). After 5 months of treatment, a repeat scan showed enlargement of the lesions, and chemotherapy was discontinued.
F. came to see me in June 1989 and entered the program; 11 months later, a CAT scan showed a 30 percent reduction in her liver tumors. In 1992 another scan showed a 95 percent reduction in her tumors. She continued to do well after 5 years on the program.
II. J., a 50-year-old businesswoman, underwent right breast lumpectomy for carcinoma in November 1986. J. did well until July 1989, when her physician detected a mass in her right breast. A lumpectomy documented poorly differentiated adenocarcinoma. An abdominal ultrasound revealed a density in the right lobe of the liver; a needle biopsy confirmed carcinoma.
J. began CMF chemotherapy, but in November 1989, after completing three cycles, she refused further treatment. At that point there had been no improvement in her liver lesions. For several months she did nothing. She then learned of my work and, in April 1990, she began the program. After two years on her protocol, she felt so well that, without my knowledge, she discontinued the protocol. In July 1991 she suffered a gran mal seizure; a CAT scan revealed two brain lesions.
J. immediately resumed her full program, and showed rapid improvement in all symptoms. CAT scans of both the head and the abdomen on April 17, 1992, were completely normal and she remains well.
III. G. is a 55-year-old woman who noticed a right breast mass which her doctor diagnosed as mastitis in mid-1984. In August 1985 her right breast suddenly enlarged; a biopsy revealed poorly differentiated adenocarcinoma and inflammatory breast disease. In September 1985 G. began radiotherapy to the chest wall; in November 1985 she underwent a right modified mastectomy. The pathology report describes carcinoma in 17 of the 17 axillary nodes. After surgery G. began chemotherapy with CMF, which she continued for 2 years. However, in August 1987 a bone scan documented increased activity in the sternum, confirmed as metastases.
G. learned of my work and began her program in December 1987. Today, after six years, she follows her nutritional regimen and is in excellent health.
http://www.alternative-doctor.com/cancer/kelley.htm
Dr. Kelley’s Do-it-Yourself Book one answer to cancer Reviewed after 32 years 1967 — 1999 By Dr. William Donald Kelley, D.D.S., M.S. 1999 THIS IS THE ENTIRE BOOK ON ONE WEB PAGE! (Keith Scott-Mumby)
http://www.alternative-doctor.com/cancer/beard.htm
John Beard’s Trophoblast Cell Theory (and a mention of its modern equivalent)
1. Beard, J: “The Action of Trypsin…” Br Med J 4, 140-41, 1906.
TRYPSIN_JENSENS_MOUSE_TUMOUR_Beard_John_JBrMed_1906
2. Beard, J: “The Enzyme Treatment of Cancer” London: Chatto and Windus, 1911. link to pdf: ENZYME_TREATMENT_CANCER_John_Beard_1911
3. Cutfield, A: “Trypsin Treatment in Malignant Disease” Br Med J 5, 525, 1907.
4. Wiggin, FH: “Case of Multiple Fibrosarcoma Of The Tongue, With Remarks on the Use of Trypsin and Amylopsin in the Treatment of Malignant Disease” JAMA 47, 2003-08. 1906.
5. Gotze, H, Rotham SS: Enterohepatic Circulation of Digestive Enzymes As A Conservative Mechanism” Nature 257 (5527).
6. Shively, FL: “Multiple Proteolytic Enzyme Therapy Of Cancer.” Dayton, Johnson-Watson, 1969.
7. Little, WL: “A Case Of Malignant Tumor, WIth Treatment.” JAMA 50, 1724, 1908.
8. Kelley, WD: “One Answer To Cancer” latest update – 33,000 cancer cases over three decades. New Century Promotions 3711 Alta Loma Drive Bonita, CA 91902 800-768-8484 or 619-479-3829.
Enzymes in Cancer Studies
http://www.medscape.com/medline/abstract/11561867?prt=true
Mixture of trypsin, chymotrypsin and papain reduces formation of metastases and extends survival time of C57Bl6 mice with syngeneic melanoma B16.
Cancer Chemother Pharmacol. 2001; 47 Suppl:S16-22 (ISSN: 0344-5704)
Wald M ; Olejár T ; Sebková V ; Zadinová M ; Boubelík M ; Poucková P
http://www.mucos.cz/eng/onko/con_onco_com.htm
references for proteolytic enzymes
Wald M, Olejár T, Poucková P, Zadinová M. Proteinases Reduce Metastatic Dissemination and Increase Survival Time in C57BI6 Mice with the Lewis Lung Carcinoma. Life Sciences 1998a; 63(17):237-243.
Wald M, Olejár T, Poucková P, Zadinová M. The influence of proteinases on in vivo blastic transformation in rat species SD/Ipcv with spontaneous lymphoblastic leukemia. British Journal of Haematology 1998b;102 (1): 294.
Wald M, Olejár T, Sebkova V, Zadinová M, Boubelík M, Pouckova P. Mixture of trypsin, chymotrypsin and papain reduces formation of metastases and extends survival time of C57BI6 mice with syngeneic melanoma B16. Cancer Chemother Pharmacol 2001;47(Suppl):S16-S22.
Wald M, Poucková P, Hloušková D, Altnerová M, Olejár T. The influence of trypsin, chymotrypsin and papain on the growth of human pancreatic adenocarcinoma transplanted to nu/nu mice. The European Journal of Cancer 1999;35(4),No. 543:148.
http://users.navi.net/~rsc/beard066.htm
MEDICAL RECORD Page 1020, [June 23, 1906] Correspondence TRYPSIN AND AMYLOPSIN IN CANCER.
http://whale.to/cancer/kelley.html
Dr. William Donald Kelley, D.D.S., M.S.
http://www.cancerdecisions.com/070202.html
Part Two of the Beard Paper Centenary
http://www.outsmartyourcancer.com/pdf/ScientificArticleForSite.PDF.pdf
The Scientific Basis Behind Alternative Cancer Treatments by Tanya Harter Pierce, MA, MFCC This is the second article in a 3-part series on alternative cancer treatments. As
mentioned in part 1
http://www.cancure.org/science_paper1.htm
Trophoblasts: On the Cause of Birth And Its Relationship to Cancer Regression
http://www.holisticjunction.com/articles/2404.html
The Cure for Cancer: Theory, History and Treatment by Owen R. Fonorow
http://www.townsendletter.com/June2003/kelleycritique0603.htm
A Critique of the Kelley Nutritional-Metabolic Cancer Program by Melina A. Roberts BSc. (Hons.) University of Waterloo, 3rd year at the Canadian College of Naturopathic Medicine
From the Townsend Letter for Doctors & Patients June 2003
http://hungerforhealth.wordpress.com/2008/05/29/
enzyme-therapy-for-cancer-prevention-and-treatment/
http://members.aol.com/pbchowka/gonzalez2002.html
One Man, Alone Dr. Nicholas Gonzalez has compelling results and a landmark grant from the National Cancer Institute. Now he just needs to convince doctors to trust him with their patients. By Peter Barry Chowka with Kathi Head, N.D. Alternative Medicine Magazine
April 2002
http://scholar.google.com/scholar?hl=en&lr=&q=related:jNPD1mSZmHcJ:scholar.google.com/
http://en.wikipedia.org/wiki/Trophoblast
Trophoblasts are invasive, eroding, and metastasizing cells of the placenta.
Trophoblasts mediate the implantation of the embryo into the endometrium, but they are never incorporated into the mother’s body or the fetus. They are not “fetal” cells.
Trophoblasts become inert during pregnancy and are completely rejected by the fetus and mother at delivery. They can be seen as the thin membrane covering the fetus at birth, the caul.[1]
http://www.cancure.org/trophoblastic_nature_of_cancer.htm
The Trophoblastic Nature of Cancer and Pregnancy Cycle as the Basis for the Enzyme Treatment of Cancer by Roger Cathey
http://www.alkalizeforhealth.net/Lstemcells.htm
Ralph W. Moss, Ph.D. Weekly CancerDecisions.com
Newsletter #81 04/26/03 Scientists Identify Stem Cells As Hidden Cause of Cancer
Al-Hajj M, et al. From the cover: prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3983-8.
University newsletter:
http://www.med.umich.edu/opm/newspage/2003/tumorsc.htm
Steinberg D. Stem cell discoveries stir debate. The Scientist 2000;14:1. Accessed at
http://www.the-scientist.com/yr2000/nov/steinberg_p1_001113.html.
Thomson JL, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7.
Goshen R, et al. Hyaluronan, CD44 and its variant exons in human trophoblast invasion and placental angiogenesis. Mol Hum Reprod. 1996;2:685-91.
U.S. Patent No. 5,843,780, “Primate embryonic stem cells”; accessible at www.uspto.gov.
Beard J. Embryological aspects and etiology of carcinoma. Lancet 1902;1:1758.
Beard J. The Enzyme Treatment of Cancer. London: Chatto & Windus, 1911.
HCG and Cancer
Acevedo HF, et al. Detection of membrane-associated human chorionic gonadotropin and its subunits on human cultured cancer cells of the nervous system. Cancer Detect Prev. 1997;21(4):295-303.
Acevedo HF and Hartsock RJ. Metastatic phenotype correlates with high expression of membrane-associated complete beta-human chorionic gonadotropin in vivo. Cancer. 1996 Dec 1;78(11):2388-99.
Acevedo HF, et al. Human chorionic gonadotropin-beta subunit gene expression in cultured human fetal and cancer cells of different types and origins. Cancer. 1995 Oct 15;76(8):1467-75.
Regelson W. Have we found the “definitive cancer biomarker”? The diagnostic and therapeutic implications of human chorionic gonadotropin-beta expression as a key to malignancy. Cancer. 1995;76:1299-301.
http://www.oasisadvancedwellness.com/health-articles/2008/06/suppression-of-alternative-cancer.html
Tuesday, June 24, 2008 The Suppression of Alternative Cancer Treatments CancerDecisions Newsletter Archives For June 22, 2008 A GREAT OPPORTUNITY LOST Ralph Moss, Ph.D
GFP
http://en.wikipedia.org/wiki/Green_fluorescent_protein
The green fluorescent protein (GFP) is a protein, composed of 238 amino acids (26.9 kDa), originally isolated from the jellyfish Aequorea victoria/Aequorea aequorea/Aequorea forskalea that fluoresces green when exposed to blue light.
http://tsienlab.ucsd.edu/Publications/Tsien%201998%20Annu.%20Rev.%20Biochem%20-%20GFP.pdf
THE GREEN FLUORESCENT PROTEIN by Roger Y. Tsien. In just three years, the green fluorescent protein (GFP) from the jellyfish Aequorea victoria has vaulted from obscurity to become one of the most widely studied and exploited proteins in biochemistry and cell biology. Its amazing ability to generate a highly visible, efficiently emitting internal fluorophore is both intrinsically fascinating and tremendously valuable. GFP has become well established as a marker of gene expression and protein targeting in intact cells and organisms. Mutagenesis and engineering of GFP into chimeric proteins are opening new vistas in physiological indicators, biosensors, and photochemical memories.
http://www.cancerimmunity.org/v7p19/071019.htm
Cancer Immunity, Vol. 7, p. 19 (6 November 2007)
Cancer is a somatic cell pregnancy. Lloyd J. Old. Ludwig Institute for Cancer Research, New York Branch at Memorial Sloan-Kettering Cancer Center, New York, NY, USA
http://www.medscape.com/viewarticle/510222?rss
From Nature Reviews Cancer. Cancer/Testis Antigens, Gametogenesis and Cancer Posted 08/12/2005 Andrew J. G. Simpson; Otavia L. Caballero; Achim Jungbluth; Yao-Tseng Chen; Lloyd J. Old Nat Rev Cancer. 2005;5(8):615-625.
http://www.ncbi.nlm.nih.gov/pubmed/11934257
Can J Physiol Pharmacol. 2002 Feb;80(2):142-9.
Human placental trophoblast as an in vitro model for tumor progression.
Lala PK, Lee BP, Xu G, Chakraborty C.
http://cancerres.aacrjournals.org/cgi/content/abstract/67/19/9528
Immunology A Placenta-Specific Gene Ectopically Activated in Many Human Cancers Is Essentially Involved in Malignant Cell Processes. Michael Koslowski1, Ugur Sahin1, Rita Mitnacht-Kraus2, Gerhard Seitz3, Christoph Huber1 and Özlem Türeci2
http://www.cancerci.com/content/5/1/4
http://www.biomedcentral.com/content/pdf/1475-2867-5-4.pdf
Cancer/testis antigens and gametogenesis: a review and “brain-storming” session Martins Kalejs* and Jekaterina Erenpreisa Cancer Cell International 2005, 5:4
http://humupd.oxfordjournals.org/cgi/reprint/dml048v1
Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. C.Ferretti et al.
Human Reproduction Update Advance Access published October 26, 2006
“…it was suggested that most and perhaps all types of human tumors share six essential alterations in cell physiology that collectively dictate malignant growth:
1) self-sufficiency in Growth Signals (GS),
2) insensitivity to growth-inhibitory (antigrowth) signals,
3) evasion of programmed cell death (apoptosis),
4) limitless replicative potential,
5) sustained angiogenesis and tissue invasion and metastasis.An overview of signaling circuitries used by trophoblast cells, although simplistic, places emphasis on the many circuitries shared with those employed by cancer cells…. it is not totally surprising that normal trophoblasts and malignant cells, which may have to accomplish comparable tasks to proliferate and migrate so as to ultimately invade neighboring tissue, use, in part, similar regulatory and effector components, similar circuitries and similar mechanisms…” quote attributed to C. Ferretti.
http://www.stopcancer.com/enzymes_wobenzym.htm
Wobenzym enzymes 1-(888) 484-8264
http:// www.wobenzym.com
http://www.oasisadvancedwellness.com/health-articles/2008/06/suppression-of-alternative-cancer.html
Tuesday, June 24, 2008 The Suppression of Alternative Cancer Treatments
http://www.ncbi.nlm.nih.gov/pubmed/9458933
Review: analogies between trophoblastic and malignant cells. Mullen CA.Am J Reprod Immunol. 1998 Jan;39(1):41-9.
Trophoblastic and malignant cells share a number of similarities. These include a lack of major histocompatibility complex antigen expression, resistance to lysis by natural killer cells, T-helper cell-2 (TH2)-biased response, prostaglandin E production, and response to transforming growth factor beta. In addition, the analogies between trophoblastic and malignant cells extend into immunotherapy in which anti-idiotype therapy has a viable role in the prevention of pregnancy loss and the treatment of cancer. CONCLUSIONS: Trophoblastic and malignant cells use a number of similar mechanisms to resist rejection by their host. By using similar strategies these cells are able to successfully co-exist in an immunologically hostile environment.
Krebs- Very Important Paper
http://www.cancergnosis.com/History/Trophablastic%20theory.pdf
THE UNITARIAN OR TROPHOBLASTIC THESIS OF CANCER
by Ernst T. Krebs, Jr.,* Ernst T. Krebs, Sr.,** and Howard H. Beard***
(Reprinted From the Medical Record, 163:149-174, July 1950)
Since the primitivity of the cancer cell is a commonplace, in looking for its cellular counterpart in the life-cycle we turn to the most primitive cell in this cycle. This is the trophoblast cell. Then as a logical corollary of the unitarian thesis, we should find the trophoblast as the constant malignant component in all exhibitions of cancer: the malignancy of the cancer varying directly with its concentration of trophoblast cells and inversely with its concentration of somatic cells.
It is, therefore, most significant that when pregnancy trophoblast is malignantly exhibited as primary uterine chorionepithelioma there is no ascertainable cytological, endocrinological or other intrinsic change whatever from the normal trophoblast cell. As Boyd has phrased it, “microscopically the chorionepithelioma is an exaggeration of the condition normally found in pregnancy.”23 All other tumors represent an attenuation of the condition of their normal tissue of origin.
The chorionepitheliomas are unquestionably the most malignant tumors in either sex, and the degree of their malignancy is routinely determined by measuring the gonadotrophin their trophoblast cells excrete.
If cancer is a truly unitarian phenomenon, then its cellular origin as well as its cellular nature are exemplified in the origin and nature of the most malignant exhibition of cancer—primary uterine chorionepithelioma.
It is thus a simple embryological fact that the malignant component of the most malignant of all exhibitions of cancer—primary uterine chorionepithelioma—represents the unchecked growth of normal trophoblast that has arisen through the differentiation of a diploid totipotent cell, by reduction division, and the division of the consequent haploid gametogenous cell to produce trophoblast. We have seen the proof of this in the fiercely malignant behavior of rabbit trophoblast removed from the checking influences of the maternal host and placed in tissue culture. This trophoblast, of course, came into being through processes normal to the production of all trophoblast in normal gestation. This is true also of the trophoblast of primary uterine chorionepithelioma.
Carcinogenesis is thus seen to be a phenomenon involving a spatially anomalous differentiation in response to organizer stimuli. (Primary uterine chorionepithelioma—as well as normal pregnancy trophoblast—while involving precisely the same differentiation in its origin does not, of course, involve it anomalously.) The differentiation involves the phenomenon of meiosis with the consequent production of trophoblast, which, presented ectopically, is inevitably exhibited as cancer—the malignancy of which depends upon the extent to which such ectopic trophoblast is resisted. Thus in the unitarian thesis we see the malignant component in all exhibitions of cancer deriving from precisely the same cell type from which the chorionepitheliomas arise. We see all producing the same cell type—trophoblast. We see this cell doing ectopically precisely what it does in its normal canalization: eroding, infiltrating, and metastasizing.
“One of the most important problems in cancer research,” Greenstein55 points out, “is concerned with the question of why primary tumors metastasize.” If cancer is trophoblastic, the problem of metastases is resolved: the normal pregnancy trophoblast is the only cell in the life-cycle that regularly metastasizes, doing so throughout the maternal host in the early months of pregnancy.56, 57
one question alone remains here: can the same diploid totipotent cell in an extragenital site undergo meiosis to eventuate in trophoblast production?
As we have seen, carcinogenesis involves ectopically precisely the same basic mechanisms involved in the production of canalized trophoblast. The prolonged exposure of a tissue to carcinogens results in a prolonged depression in its respiratory mechanisms.125 This may result in the appearance and persistence of ectopic trophoblast in the exposed tissue. The trophoblast or cancer cell is autonomous of the hostal respiratory system and is obligatively anaerobic, undergoing [an]aerobic glycolysis even in the presence of a free oxygen supply.126 The trophoblastic thesis explains the long-known identity of trophoblast cell metabolism with that of the cancer cell: 127, 128, 129 an obligative anaerobic system is obviously a necessity in a primitive parasitic cell like the trophoblast (or cancer) cell.
As a composite tissue, cancer in its somatic component represents many diseases; in its constant malignant component, one disease; and, in its totality, a local manifestation of a general disease. Since the perspective of the clinician is necessarily anthropomorphic, he sees cancer primarily in its somatic phase as a series of many diseases. On the other hand, as Oberling and Woglom have so aptly phrased it, “To the experimentalist cancer is one disease and one disease only.”
What are the factors—cells, tissues, organs, and their secretions—contributing to such resistance? What causes trophoblast in the pregnant diabetic to overgrow, despite a normal insulin supplement? Why do the specific inhibitors to pancreatic chymotrypsin and trypsin rise with the increasing malignancy of a growth and decline following its amelioration? Why is the small intestine practically immune not only to primary tumors, but to direct invasion and metastases as well? Why does the growth of the invasive, erosive and metastatic trophoblast of normal gestation cease and degeneration commence concomitant with the commencing function of the fetal pancreas gland? Why does the urinary excretion of chorionic gonadotrophin fall concomitantly with the degeneration of the trophoblast? After more than 99 per cent of the trophoblast has been removed from the placenta, why does its size remain unaffected though its invasive and erosive properties are entirely lost? Why are pregnancy trophoblast cells often indistinguishable histologically from the somatic cells in the uterine wall of the pregnant host? Why is it that the removal of normal pregnancy trophoblast to tissue culture will result in a fiercely malignant exhibition of such trophoblast toward all nontrophoblast cells?*
*In reviewing over 17,000 papers on cancer and related biological subjects the senior author, in the course of his text on “The Biological Basis of Cancer,” has not found a single valid contribution that fails to find congruence with, and illumination from the trophoblastic or unitarian thesis of cancer.
http://users.navi.net/~rsc/thesis.htm
On the Cause of Birth and Its Relation to Cancer Regression. Roger S. Cathey Updated July 12, 2003
A simple search of Medline with just two terms: trophoblast and cancer will return some 50 pages of 20 citations per page, and the number of studies and observations is always increasing. Not by any means focusing exclusively on gestational cancers. Collectively, these studies and especially because of the genetic motifs they have revealed compel the conclusion that cancers of all types, regardless of origin are trophoblastic.
This paper examines these questions and proposes that the cause of birth and the regression of cancer may be
A) mediated not by intrinsic genetic programs (within the trophoblast), but by extrinsic, “epigenetic” factors akin to immunologic processes.
![]() that these anti-malignant processes consist primarily in enzymatic digestion after enzyme inhibition is overcome.
that these anti-malignant processes consist primarily in enzymatic digestion after enzyme inhibition is overcome.
C) that the enzyme expression effecting these changes is primarily a combined assault from the pancreases of both mother and fetus, therefore making the pancreas a component of the immune system, and
D) that the normal cellular immune components of the maternal system are potentiated by this prior maternal-fetal pancreatic enzymatic attack.
The tumor or placenta responds to enzyme attack by producing multiple nuclei both by cellular fusion, and nuclear division, for the sake of greater transcription to replace the continuously digested cell coats at the limb of the placenta or the cancer tumor. In addition to multiplication of nuclei, it appears that by means of desmosomes or gated channels that this form of trophoblast may take on characteristics of the cells with which they fuse that are entirely normal until such hybridization. At this point it may be that we see the appearance of odd numbers of genes, pleuripoidy, anuploidy, etc. as if the syncytial phase — which we might term the defensive and relatively benign, or non-metatstatic phase of “trophoblastism” — takes selected components or gene-sectors and multiplies them. This is the source of the seemingly unlimited variety of cancers that exist.
Hypoxia
http://lib.bioinfo.pl/pmid:15141079/pmid/cit
Mol Biol Cell. 2005 Apr ;16 (4):1901-12
Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression.
Karen D Cowden Dahl, Sarah E Robertson, Valerie M Weaver, M Celeste Simon
Hypoxia-inducible factor (HIF)-deficient placentas exhibit a number of defects, including changes in cell fate adoption, lack of fetal angiogenesis, hypocellularity, and poor invasion into maternal tissue. HIF is a heterodimeric transcription factor consisting of alpha and beta aryl hydrocarbon receptor nuclear translocator or ARNT) subunits. We used undifferentiated trophoblast stem (TS) cells to characterize HIF-dependent adhesion, migration, and invasion. Arnt(-/-) and Hifalpha(-/-) TS cells exhibit reduced adhesion and migration toward vitronectin compared with wild-type cells. Furthermore, this defect is associated with decreased cell surface expression of integrin alphavbeta3 and significantly decreased expression of this integrin in focal adhesions. Because of the importance of adhesion and migration in tumor progression (in addition to placental development), we examined the affect of culturing B16F0 melanoma cells in 1.5% oxygen (O(2)). Culturing B16F0 melanoma cells at 1.5% O(2) resulted in increased alphavbeta3 integrin surface expression and increased adhesion to and migration toward vitronectin. Together, these data suggest that HIF and O(2) tension influence placental invasion and tumor migration by increasing cell surface expression of alphavbeta3 integrin.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1073670
Mol Biol Cell. 2005 April; 16(4): 1901–1912.
Hypoxia-inducible Factor Regulates αvβ3 Integrin Cell Surface Expression
Karen D. Cowden Dahl,* Sarah E. Robertson,† Valerie M. Weaver,‡§ and M. Celeste Simon*
Molecular Biology shows Trophoblast = cancer
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=407853
Reprod Biol Endocrinol. 2004; 2: 15.
Trophoblast ‘pseudo-tumorigenesis’: Significance and contributory factors
Rama Soundararajan1 and A Jagannadha Rao1,2
Trophoblast cells of the human placenta proliferate, migrate, and invade the pregnant uterus and its vasculature in order to nourish the developing fetus, in a way that is imitated by malignant tumors. Many similarities exist between embryo implantation and the growth of cancer cells. We begin this article by reviewing decades of studies that have helped unearth the mechanisms that contribute to the tumor-like phenotype of human trophoblast cells. Interestingly, these attributes are only transient in nature, with stringent spatial and temporal confines. The importance of intrinsic molecular controls that effectively circumscribe the extent and duration of trophoblast incursion, becomes increasingly evident in abnormal pregnancies that are characterized by aberrant trophoblast proliferation/invasion. We summarize and discuss the significance of abnormalities in these regulatory mechanisms, and finally, speculate about the use of human trophoblastic cells as model systems for the study of a variety of cellular processes. While on one hand, human placental cells are bestowed with a capacity to proliferate indefinitely and invade extensively, on the other, these cells are also replete with mechanisms to regulate these tumor-like attributes and eventually progress to a senescent apoptotic state. This is therefore, a ‘well-behaved’ tumor. The comparison in the present review is between the invasive cytotrophoblastic cell type and the tumor cell type.
The normal human cytotrophoblast expresses functional tumor-associated genes, which are essential prerequisites for a malignant phenotype. The study of the control of these genes in normal cells, or in the physiological context, may therefore, reveal a basis for the future treatment of cancer.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=15372095
Trophoblast Differentiation During Embryo Implantation and Formation of the Maternal-Fetal Interface
October 2004
Oxygen regulates trophoblast differentiation and proliferation. Physiological factors also play an important role in formation of the maternal-fetal interface and, consequently, fetal growth. Oxygen tension, a function of uterine blood flow, is a prime example (S11). In recent years a great deal has been learned about the fundamental mechanisms that couple the trophoblasts’ ability to sense oxygen levels with their differentiative and metabolic status. Important clues about oxygen’s effects on the placenta have come from several lines of evidence that suggest that the early stages of placental (and embryonic) development take place in an environment that is hypoxic relative to the uterus. Specifically, direct measurement of uterine oxygen tension demonstrates physiological hypoxia (2-5% O2; reviewed in ref. 100). Furthermore, blood flow to the human intervillous space does not begin until 10 to 12 weeks of pregnancy (101). Studies in both nonhuman primates (S16) and humans (102) suggest that trophoblasts actively limit their access to uterine blood by plugging the lumina of the decidual vessels. Why? Our work shows that cytotrophoblasts proliferate in vitro under hypoxic conditions that are comparable to those found during early pregnancy in the uterine cavity and the superficial decidua. As trophoblast invasion of the uterus proceeds, the placental cells encounter increasingly higher oxygen levels, which trigger their exit from the cell cycle and subsequent differentiation (103, 104). Hypoxia also regulates cell fate in the niurine placenta (105). We speculate that the paradoxical effects of oxygen in controlling the balance between cytotrophoblast proliferation and differentiation explain in part why the mass of the placenta increases much more rapidly than that of the embryo. Histological sections of earlystage pregnant human uteri show bilaminar embryos surrounded by thousands of trophoblast cells (S17). The fact that hypoxia stimulates cytotrophoblasts, but not most other cells, to undergo mitosis (106) could help account for the difference in size between the embryo and the placenta, a discrepancy that continues well into the second trimester of pregnancy (S18). Thus, the structure of the mature placenta is established in advance of the period of rapid fetal growth that occurs during the latter half of pregnancy.
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=17288592
Control of human trophoblast function
Laura Lunghi,1 Maria E Ferretti,1 Silvia Medici,1 Carla Biondi,1 and Fortunato Vesce2
A relatively low oxygen environment characterizes blastocyst implantation, placentation and early embryonic development up to the 10th week of gestation [29]. Accordingly, in the first trimester, the villous trophoblastic layer is twice the thickness it becomes in the second. Moreover, the intervillous circulation is established peripherically at around the 9th week, and it enlarges to encompass the whole placenta only after the 12th week. This could be due to the presence of trophoblast cell plugs which occlude the tips of the uteroplacental arteries [30], or to incomplete spiral artery remodeling [31]. Low O2 levels stimulate CT proliferation and inhibit EVT and ST differentiation. Hypoxia acts by modifying gene expression or mRNA stability [32]. For instance it stimulates the expression of both the hypoxia inducible factor-1 (HIF-1), which maintains CT proliferation, and Hash-2, thus preventing differentiation. Moreover it has been reported that, in first-trimester trophoblast, HIF-1 expression parallels that of TGF-β3, an inhibitor of early trophoblast differentiation which impairs its acquisition of the invasive phenotype [31,33]. In line with this last report, it has been observed that invading CT downregulates the expression of both HIF-1 and TGF-β3 genes [33,34]. cAMP also seems to be involved in trophoblast differentiation and it has been shown to influence ST formation. It enhances AP-2 and Sp functionality, promotes the expression of the syncytin gene, a highly fusogenic membrane protein localized at the CT-ST interface, and mediates the syncytialization evoked by CG [35]. Among growth factors, the vascular endothelial growth factor (VEGF), whose expression has been demonstrated in decidual cells, CT and EVT [36-38], stimulates CT differentiation to both ST and endovascular EVT, and its effect is inhibited by soluble fms-like tyrosine kinase-1 (sFlt-1), which acts by binding VEGF [39].
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1800852
Reprod Biol Endocrinol. 2007; 5: 6.
Hypoxia
http://www.ncbi.nlm.nih.gov/pubmed/16234296
Hum Reprod Update. 2006 Mar-Apr;12(2):137-44. Epub 2005 Oct 18. Links
The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy.James JL, Stone PR, Chamley LW.
In the first trimester of human pregnancy villous cytotrophoblasts are able to differentiate to form either the overlying syncytiotrophoblast layer or, in anchoring villi, extravillous trophoblasts which grow out from the villi and invade into the maternal decidua, acting to both physically attach the placenta to the decidua, and modify the maternal spiral arteries to sustain pregnancy. During the first 10-12 weeks of gestation, extravillous trophoblast plugs block the spiral arteries and prevent maternal blood flow entering the intervillous space, thereby creating an environment of physiological hypoxia in which placental and fetal development occur. As extravillous trophoblasts migrate away from the villus they differentiate from a proliferative to an invasive phenotype. The hypoxic environment of the first trimester is believed to play an important role in the regulation of trophoblast differentiation. However, there is currently a large body of conflicting experimental evidence concerning this topic. This review examines the experimental evidence to date on the role of oxygen in trophoblast differentiation.
Aneuploidy – Confined Placental Mosaicism
Comparative genomic hybridization:
A new approach to screening for intrauterine complete or mosaic aneuploidy
S. Lestou 1, V. Desilets 2 3, B.L. Lomax 1, I.J. Barrett 1, R.D. Wilson 2, S. Langlois 3, D.K. Kalousek 1 3 *
Abstract
In the practice of clinical genetics chromosomal aneuploidy in both mosaic and nonmosaic forms has long been recognized as a cause of abnormal prenatal and postnatal development. Traditionally, cytogenetic analysis of cultured lymphocytes has been used as a standard test for detection of constitutional aneuploidies. As lymphocytes represent only one lineage, chromosomal mosaicism expressed in other tissues often remains undetected. The purpose of this study was to assess the utilization of molecular cytogenetic analysis for detection of chromosomal aneuploidy in placental tissues. Using placentas from 100 pregnancies with viable nonmalformed livebirths, both trophoblast and chorionic stroma were analyzed using comparative genomic hybridization (CGH). In all cases with an indication of chromosomal imbalance by CGH, fluorescence in situ hybridization (FISH) analysis was performed to confirm the presence of aneuploidy. To differentiate between constitutional aneuploidy and confined placental mosaicism (CPM), amniotic membrane was analyzed by CGH and FISH techniques. Our results demonstrated five placentas with CPM for chromosomes 2, 4, 12, 13, and 18, respectively, and two constitutional nonmosaic aneuploidies (47,XXX and 47,XXY). Molecular cytogenetic studies of human placental tissues enables easy analysis of both embryonic (amnion) and extraembryonic (chorion) cell lineages. Detection at birth of chromosomal defects affecting intrauterine placental and fetal development is important because these chromosomal defects may continue to have an influence on postnatal development. Am. J. Med. Genet. 92:281-284, 2000. © 2000 Wiley-Liss, Inc.
http://en.wikipedia.org/wiki/Confined_placental_mosaicism
Confined placental mosaicism
From Wikipedia, the free encyclopedia
Confined placental mosaicism (CPM) represents a discrepancy between the
chromosomal makeup of the cells in the placenta and the cells in the baby.
CPM was first described by Kalousek and Dill in 1983.
CPM is diagnosed when some trisomic cells are detected on chorionic villus sampling
and only normal cells are found on a subsequent prenatal test,
such as amniocentesis or fetal blood sampling.
In theory, CPM is when the trisomic cells are found only in the placenta.
CPM is detected in approximately 1-2% of ongoing pregnancies that are studied by
chorionic villus sampling (CVS) at 10 to 12 weeks of pregnancy.
Chorionic villus sampling is a prenatal procedure which involves a placental biopsy.
Most commonly when CPM is found it represents a trisomic cell line in the placenta and a normal diploid chromosome complement in the baby (Robinson et al, 1997). However, the fetus is involved in about 10% of cases (Phillips et al, 1996)
http://www.sciencemag.org/cgi/content/abstract/221/4611/665
Science 12 August 1983:
Vol. 221. no. 4611, pp. 665 – 667
Chromosomal mosaicism confined to the placenta in human conceptions
DK Kalousek and FJ Dill
Placental and fetal tissues from 46 human pregnancies were cultured and cytogenetically analyzed in an attempt to document the existence of chromosomal mosaicism confined strictly to tissues of extraembryonic origin. In two gestations in which chromosomal mosaicism was found, it was expressed exclusively in placental chorionic cells and was not detected in cells derived from the embryo proper. This demonstration of confined chorionic mosaicism may have implications for the understanding of the fetoplacental unit and for prenatal diagnosis.
http://www.ncbi.nlm.nih.gov/pubmed/16333823
Am J Med Genet A. 2006 Jan 1;140(1):24-30. Links
Investigation of confined placental mosaicism (CPM) at multiple sites in post-delivery placentas derived through intracytoplasmic sperm injection (ICSI).Minor A, Harmer K, Peters N, Yuen BH, Ma S.
Department of Obstetrics and Gynecology, University of British Columbia, 855 West 12th Avenue, Vancouver, British Columbia V5Z 1M9, Canada.
Although earlier studies on pregnancies derived through intracytoplasmic sperm injection (ICSI) reported increased non-mosaic aneuploidy among ICSI children, undetected mosaicism, such as confined placental mosaicism (CPM) has not been evaluated. We investigated the incidence of CPM in post-delivery placentas derived from ICSI, evaluated whether CPM was increased and whether it was a contributing factor to negative pregnancy outcome. [Fifty-one post-delivery placentas were collected from patients who underwent ICSI with a normal or negative pregnancy outcome]. Trophoblast and chorionic stroma from three sites were analyzed by comparative genomic hybridization (CGH) and flow cytometry. Detected abnormalities were confirmed by fluorescence in situ hybridization (FISH). The incidence of CPM in the ICSI population was compared to the general population from published data. We detected three cases of CPM in our study. One abnormality was found by CGH analysis; partial trisomy 7q and a partial monosomy Xp limited to the trophoblast at two sites. The abnormality was associated with a child affected by spina bifida. Two cases of mosaic tetraploidy were observed by flow cytometry in pregnancies with a normal outcome. All three abnormalities were confirmed by FISH analysis. The incidence of CPM in the ICSI study population was 5.88% (3/51), which was not statistically different from published reports in the general population (5.88% (42/714), Chi square, P > 0.05). The post-ICSI population was not at risk for CPM in this study. (c) 2005 Wiley-Liss, Inc.
http://www.ncbi.nlm.nih.gov/pubmed/16274964
Mech Dev. 2005 Dec;122(12):1266-81. Epub 2005 Nov 4. Links
Fate of tetraploid cells in 4n<–>2n chimeric mouse blastocysts.Mackay GE, West JD.
Division of Reproductive and Developmental Sciences, Genes and Development Group, University of Edinburgh, Hugh Robson Building, George Square, Edinburgh EH8 9XD, Scotland, UK.
Previous studies have shown that tetraploid (4n) cells rarely contribute to the derivatives of the epiblast lineage of mid-gestation 4n<–>2n mouse chimeras. The aim of the present study was to determine when and how 4n cells were excluded from the epiblast lineage of such chimeras. The contributions of GFP-positive cells to different tissues of 4n<–>2n chimeric blastocysts labelled with tauGFP were analysed at E3.5 and E4.5 using confocal microscopy. More advanced E5.5 and E7.5 chimeric blastocysts were analysed after a period of diapause to allow further growth without implantation. Tetraploid cells were not initially excluded from the epiblast in 4n<–>2n chimeric blastocysts and they contributed to all four blastocyst tissues at all of the blastocyst stages examined. Four steps affected the allocation and fate of 4n cells in chimeras, resulting in their exclusion from the epiblast lineage by mid-gestation. (1) Fewer 4n cells were allocated to the inner cell mass than trophectoderm. (2) The blastocyst cavity tended to form among the 4n cells, causing more 4n cells to be allocated to the hypoblast and mural trophectoderm than the epiblast and polar trophectoderm, respectively. (3) 4n cells were depleted from the hypoblast and mural trophectoderm, where initially they were relatively enriched. (4) After implantation 4n cells must be lost preferentially from the epiblast lineage. Relevance of these results to the aetiology of human confined placental mosaicism and possible implications for the interpretation of mouse tetraploid complementation studies of the site of gene action are discussed.
Evidence of placental metastases
http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1735725&blobtype=pdf
Detection of cell free placental DNA in maternal plasma:direct evidence from three cases of confined placental mosaicism. H Masuzaki, K Miura, K-i Yoshiura, S Yoshimura, N Niikawa, T Ishimaru J Med Genet 2004;41:289–292. doi: 10.1136/jmg.2003.015784
http://www.ncbi.nlm.nih.gov/pubmed/11973523
J Gynecol Obstet Biol Reprod (Paris). 2002 Feb;31(1 Suppl):2S70-4.
[Confined placental mosaicism: definition, consequences and outcome][Article in French]
Viot G.
Confined placental mosaicism (CPM) occurs in approximately 2 per cent of viable pregnancies studies by chorionic villus sampling. The great majority of pregnancies with confined placental mosaicism proceed uneventfully, resulting in normal liveborn infants. However, a few specific chromosomal abnormalities have been associated with fetal growth retardation. Fetal karyotype should be checked by amniocentesis and such pregnancies should have careful ultrasound follow-up.
http://www.ncbi.nlm.nih.gov/pubmed/8338621
Fetal Diagn Ther. 1993 Mar-Apr;8(2):102-8.Links
Viable pregnancies after diagnosis of trisomy 16 by CVS: lethal aneuploidy compartmentalized to the trophoblast.
Johnson MP, Childs MD, Robichaux AG 3rd, Isada NB, Pryde PG, Koppitch FC 3rd, Evans MI.
Increasing utilization of chorionic villus sampling (CVS) has lead to the discovery that the placenta can karyotypically be a very heterogeneous organ, and chromosomal mosaicism within the placental can confuse cytogenetic interpretation. Recently, confined placental mosaicism (confined regions of aneuploidy in the otherwise normal diploid placental and fetus) has been described involving a number of chromosomal abnormalities. Fetal trisomy 16 is considered uniformly lethal early in gestation. However, we present 3 cases of nonmosaic trisomy 16 confined regionally to the placenta. We discuss the possible etiology, impact on the developing fetus, and suggest an approach to the workup and evaluation of cases where the karyotype obtained on CVS is not compatible with the findings on ultrasound.
_______________________________
Warburg Effect New agents to Inhibit Glycolysis
Otto Warburg
http://www.whale.to/a/warburg.html
The Prime Cause and Prevention of Cancer with two prefaces on prevention
http://healingtools.tripod.com/primecause2.html
Revised lecture at the meeting of the Nobel-Laureates on June 30, 1966
at Lindau, Lake Constance, Germany by Otto Warburg
Director, Max Planck-Institute for Cell Physiology, Berlin-Dahlem
English Edition by Dean Burk National Cancer Institute, Bethesda, Maryland, USA
The Second Revised Edition Published by Konrad Triltsch, Würzburg, Germany
1969 Preface to the Second Revised German Edition of the Lindau Lecture
(The way to prevention of cancer)
http://en.wikipedia.org/wiki/Warburg_hypothesis
Warburg hypothesis From Wikipedia, the free encyclopedia
http://www.nature.com/bjc/journal/v87/n7/abs/6600547a.html
Experimental Therapeutics British Journal of Cancer (2002) 87, 805-812.
Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death
R L Aft, F W Zhang and D Gius
http://lib.bioinfo.pl/pmid:17879147
J Bioenerg Biomembr. 2007 Sep 19; : 17879147
Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen.
Warburg who died 1 year later in 1970 had shown in the 1920s that the most striking biochemical phenotype of cancers is their aberrant energy metabolism. Unlike normal tissues that derive most of their energy (ATP) by metabolizing the sugar glucose to carbon dioxide and water, a process that involves oxygen-dependent organelles called “mitochondria”, Warburg showed that cancers frequently rely less on mitochondria and obtain as much as 50% of their ATP by meta
This frequent phenotype of cancers became known as the “Warburg effect”, and the author of this review strongly believed its understanding would facilitate the discovery of a cure. Following in the final footsteps of Warburg and caught in the midst of an unpleasant anti-Warburg, anti-metabolic era, the author and his students/collaborators began quietly to identify the key molecular events involved in the “Warburg effect”. Here, the author describes via a series of sequential discoveries touching five decades how despite some impairment in the respiratory capacity of malignant tumors, that hexokinase 2 (HK-2), its mitochondrial receptor (VDAC), and the gene that encodes HK-2 (HK-2 gene) play the most pivotal and direct roles in the “Warburg effect”. They discovered also that like a “Trojan horse” the simple lactic acid analog 3-bromopyruvate selectively enters the cells of cancerous animal tumors that exhibit the “Warburg effect” and quickly dissipates their energy (ATP) production factories (i.e., glycolysis and mitochondria) resulting in tumor destruction without harm to the animals.
http://en.wikipedia.org/wiki/3-bromopyruvate
Bromopyruvic acid From Wikipedia, the free encyclopedia
(Redirected from 3-bromopyruvate)
http://www.ncbi.nlm.nih.gov/pubmed/11578813
Ko YH, Pedersen PL, Geschwind JF (2001). “Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase”. Cancer Lett. 173 (1): 83–91. PMID 11578813.
http://www.ncbi.nlm.nih.gov/pubmed/17879147
Pedersen PL (2007). “Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen”. doi:10.1007/s10863-007-9094-x. PMID 17879147.
http://www.ncbi.nlm.nih.gov/pubmed/15465013
Ko YH, Smith BL, Wang Y, et al (2004). “Advanced cancers:
eradication in all cases using 3-bromopyruvate therapy to deplete ATP”. Biochem. Biophys. Res. Commun. 324 (1): 269–75.
http://www.ncbi.nlm.nih.gov/pubmed/17404823
J Bioenerg Biomembr. 2007 Feb;39(1):1-12. Links
The cancer cell’s “power plants” as promising therapeutic targets: an overview.
Pedersen PL.
This introductory article to the review series entitled “The Cancer Cell’s Power Plants as Promising Therapeutic Targets” is written while more than 20 million people suffer from cancer. It summarizes strategies to destroy or prevent cancers by targeting their energy production factories, i.e., “power plants.” All nucleated animal/human cells have two types of power plants, i.e., systems that make the “high energy” compound ATP from ADP and P( i ). One type is “glycolysis,” the other the “mitochondria.” In contrast to most normal cells where the mitochondria are the major ATP producers (>90%) in fueling growth, human cancers detected via Positron Emission Tomography (PET) rely on both types of power plants. In such cancers, glycolysis may contribute nearly half the ATP even in the presence of oxygen (“Warburg effect”). Based solely on cell energetics, this presents a challenge to identify curative agents that destroy only cancer cells as they must destroy both of their power plants causing “necrotic cell death” and leave normal cells alone. One such agent, 3-bromopyruvate (3-BrPA), a lactic acid analog, has been shown to inhibit both glycolytic and mitochondrial ATP production in rapidly growing cancers (Ko et al., Cancer Letts., 173, 83-91, 2001), leave normal cells alone, and eradicate advanced cancers (19 of 19) in a rodent model (Ko et al., Biochem. Biophys. Res. Commun., 324, 269-275, 2004). A second approach is to induce only cancer cells to undergo “apoptotic cell death.” Here, mitochondria release cell death inducing factors (e.g., cytochrome c). In a third approach, cancer cells are induced to die by both apoptotic and necrotic events. In summary, much effort is being focused on identifying agents that induce “necrotic,” “apoptotic” or apoptotic plus necrotic cell death only in cancer cells. Regardless how death is inflicted, every cancer cell must die, be it fast or slow.
http://cancerres.aacrjournals.org/cgi/content/full/62/14/3909
fulll text
Geschwind JF, Ko YH, Torbenson MS, Magee C, Pedersen PL (2002).
“Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production”. Cancer Res. 62 (14): 3909–13. PMID 12124317.
http://www.kosen21.org/upload_repository2/community/01230411451209597a.pdf
full text REVIEW Glycolysis inhibition for anticancer treatment
H Pelicano1, DS Martin2,{, R-H Xu3 and P Huang1 Oncogene (2006) 25, 4633–4646
Increase of aerobic glycolysis in cancer
The phenomenon of aerobic glycolysis increase in cancer cells was first described by Otto Warburg (1930) over 70 years ago. He showed that compared to normal cells, malignant cells exhibit significantly elevated glycolytic activity even in the presence of sufficient oxygen, and considered this phenomenon as the most fundamental metabolic alteration in malignant transformation, or ‘the origin of cancer cells’ (Warburg, 1956). Although the cause–effect relationship between the increase in aerobic glycolysis and the development of cancer is controversial (Zu and Guppy, 2004), increased glycolysis has been consistently observed in many cancer cells of various tissue origins (for a review, see Semenza et al., 2001), suggesting that this metabolic alteration is common in cancer. Indeed, the positron emission tomography (PET) widely used in clinical diagnosis of cancer is based on the fact that cancer cells are highly glycolytic and actively uptake glucose.
The Warburg effect can be viewed as a prominent biochemical symptom of cancer cells that reflects a fundamental change in their energy metabolic activity.
http://www.hopkinskimmelcancercenter.org/news/details.cfm?documentid=673
FOR IMMEDIATE RELEASE, OCTOBER 14, 2004
Audio file of Peter Pedersen, Ph.D., discussing the success in treating advanced liver cancers in rats.
“ENERGY BLOCKER” KILLS BIG TUMORS IN RATS
http://www.kjronline.org/abstract/view_articletext.asp?year=2007&page=216
FDG-PET for Evaluating the Antitumor Effect of Intraarterial 3-Bromopyruvate Administration in a Rabbit VX2 Liver Tumor Model
Hee Sun Park, MD, Jin Wook Chung, MD, Hwan Jun Jae, MD, Young Il Kim, MD, Kyu Ri Son, MD, Min Jong Lee, MD, Jae Hyung Park, MD, Won Jun Kang, MD, Jung Hwan Yoon, MD, PhD, Hesson Chung, PhD, Kichang Lee, DVM, PhD
Korean Journal of Radiology; 2007 June; 8(3):216-224
http://acs.confex.com/acs/norm07/techprogram/P44814.HTM
June 20, 2007
Glycolytic enzyme inhibitors as novel anti-cancer drugs. James C.K. Lai1, Vikas Bhardwaj2, Nisha Rizvi2, Tanushree Chatterji2, Alfred O. Isaac2, Maria B. Lai2, Tara Johnson2, Solomon W. Leung3, Christopher K. Daniels2, and Alok Bhushan1.
According to the Warburg hypothesis, tumor cell survival and proliferation critically depend on aerobic glycolysis rather than mitochondrial glucose oxidative metabolism as energy source. To further test the generality of the Warburg hypothesis, we have systematically investigated the effects of two glycolytic enzyme inhibitors (namely, 3-bromopyruvate (3BP), an inhibitor of hexokinase II, and iodoacetate (IAA), an inhibitor of glyceraldehyde-3-phosphate dehydrogenase) on the survival of several different types of cancer cells, including glioblastoma, pancreatic, and oral cancer cells. Our results demonstrate that both 3BP and IAA induced decreases in cell survival in all cancer cell types studied in a concentration- and time-related manner. Moreover, we found that one mechanism by which IAA induced cell death was necrosis, as determined by lactate dehydrogenase release into the medium, a marker of necrotic cell damage/death. Moreover, IAA was more potent than 3BP in inducing cell death in all the cancer cell types investigated. Thus, the results of our ongoing systematic studies prompted us to hypothesize that we can employ glycolytic enzyme inhibitors, such as IAA and 3BP, as “proof-of-concept” test drugs to derive a novel approach to inhibit cancer cell proliferation and invasion. We are currently conducting additional mechanistic and signaling studies to systematically test this hypothesis further.
Insulin Potentiated Therapy Hypoglycemia
http://www.iptq.com/
Insulin Potentiation Therapy (IPT)
http://www.contemporarymedicine.net/pub06_insulin_chemotherapy.htm
Insulin, Chemotherapy and the Mechanisms of Malignancy: The Design and the Demise of Cancer
S.G. Ayre, D.P. Garcia y Bellon, D.P. Garcia Jr
Medical Hypotheses (2000) 55(4), 330-334
Of some interest is another example of metabolic modification discussed in two obscure reports of complete cancer remissions produced by insulin-induced hypoglycemia alone – without any chemotherapy at all (50,51). These cases involved protocols very different from our own with respect to doses and timing of insulin administration, and the control of the hypoglycemia. One of the authors here posited that an accumulated hyperoxidation of the blood was responsible for the remissions. This accumulation was thought to be due to decreased oxygen needs for metabolizing glucose on account of the decreased amount of glucose in the blood. From the classical work of Warburg, it is known that cancer cell metabolism relies on the anaerobic degradation of glucose (52). Perhaps the increased tissue oxygen tension, in combination with a lowered blood glucose concentration, sufficiently perturbed cancer cell metabolism to producing the observed lethal effects. Whether these phenomena may play a role to enhance anticancer drug cytotoxicity in IPT is not known, but the possibilities are interesting.
Ayre SG, Perez Garcia y Bellon D, Perez Garcia D Jr. Insulin potentiation therapy: a new concept in the management of chronic degenerative disease. Med Hypotheses 1986;20(2):199-210
Ayre SG, Garcia y Bellon DP, Garcia DP Jr. Insulin, chemotherapy, and the mechanisms of malignancy: the design and the demise of cancer. Med Hypotheses 2000;55(4):330-4
Lasalvia-Prisco E, Cucchi S, Vazquez J et al. Insulin-induced enhancement of antitumoral response to methotrexate in breast cancer patients. Cancer Chemother Pharmacol 2004;53(3):220-4
http://www.ncbi.nlm.nih.gov/pubmed/14458502
Koroljow, S. Two cases of malignant tumors with metastases apparently treated successfully with hypoglycemic coma. Psychiatric Quarterly 1962; 36(1):261-270.
http://www.ncbi.nlm.nih.gov/pubmed/14479168
Neufeld, O. Insulin therapy in terminal cancer: a preliminary report. J Amer Geriatric Soc 1962; 10(3):274-6.
Warburg O. The metabolism of carcinoma cells. J Cancer Res 1925; 9:148-163.
A recording of Dr. Gonzalez’s speech at Boulderfest 2008, sponsored by Crayhon Research, is now available at the following link:http://www.newspringpress.com/lectures.html
Link to this article:http://wp.me/P3gFbV-yO
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Florida 33314
954-792-4663
http://www.jeffreydach.com/
http://www.drdach.com/
http://www.naturalmedicine101.com/
http://www.truemedmd.com/
http://www.bioidenticalhormones101.com/
Disclaimer click here: http://www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship.
Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2014 Jeffrey Dach MD All Rights Reserved
This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Nicholas Gonzalez, MD, and the Trophoblastic Theory of Cancer August 5, 2013 at 10:09 PM
[…] an extensive list of References go to the original article location on Dr. Jeffrey Dach’s […]
How Does Cannabis Kill Cancer Cells ? October 13, 2014 at 10:13 AM
[…] Cancer cells may produce their own growth factors as well as receptors for these growth factors, creating a powerful feedback loop for uncontrolled cell growth and replication. Cancer cells may produce proteolytic enzymes (such as matrix metalloproteases, MMPs) which dissolve the extracellular tissue matrix and allow cancer cells to invade surrounding tissues, blood vessels and lymphatic which are the gateways to metastatic spread. This is described in my previous article on the Trophoblastic theory of Cancer. […]
Cancer as a Metabolic Disease by Jeffrey Dach MD - Jeffrey Dach MD January 9, 2015 at 3:16 PM
[…] Nicholas Gonzalez and the Trophoblast Theory of Cancer […]