 Low Dose Naltrexone – LDN Part Four
Low Dose Naltrexone – LDN Part Four
by Jeffrey Dach MD
Reflex Sympathetic Dystrophy RSD – and Fibromyalgia
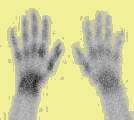
Left Image: Bone Scan showing increased uptake left hand, positive for reflex sympathetic dystrophy, courtesy of medscape.com.
My previous articles on LDN discuss its use in Multiple Sclerosis (1,2) and Crohn’s Disease (3,4). This article deals with the use of LDN for Chronic Pain Syndromes of Fibromyalgia and Reflex Sympathetic Dystrophy, RSD.
Reflex Sympathetic Dystrophy, RSD, is a neurological disorder in which a minor injury to an extremity leads to a chronic and debilitating pain syndrome called Allodynia, a form of hypersensitivity to touch. Any minor brushing or touching the skin triggers a severely painful sensation which may in turn trigger muscle spasms. The sensory-motor feedback loop in the spinal cord is magnified. Typically, there is also disturbance of the sympathetic nervous system with increased vascular flow, discoloration and swelling of the extremity.
RSD Case Report
I recall one young female who had RSD of the leg. Many years ago, her leg had been struck by a car while she was a pedestrian. The injury was minor and she recovered quickly. However, there was residual neurologic damage. She had “allodynia”, severe pain would result from a light touch to the skin, and the leg immediately went into muscle spasms. Any minimal sensory input was magnified in a feedback loop to the motor neurons system which caused excessive uncontrollable muscle movements and spasms. She had been to numerous doctors, clinics, rehabilitation facilities, and nothing they did could halt the progression of the symptoms. The doctors had given her various psychoactive drugs and increasing doses of narcotic pain killers, providing some temporary relief, yet none was effective long term. She was now confined to a wheel chair and addicted to narcotics. Her future appeared bleak. However she had not yet been treated with LDN, and this was worth a try.
Low Dose Naltrexone is a capsule taken at night before sleep. Except for the fact that it is a narcotics blocker, there are no known adverse effects. Habitual users of narcotic pain pills (oxycodone, morphine, tramadol) will be thrown into drug withdrawal if they take LDN, so it is not advised for this group.
Radionuclide Bones Scans
 Decades ago when I was a nuclear medicine resident in training, we did radionuclide bone scans on the RSD patients. The diagnosis was made by increased uptake in the affected extremity (left image).(16-18)
Decades ago when I was a nuclear medicine resident in training, we did radionuclide bone scans on the RSD patients. The diagnosis was made by increased uptake in the affected extremity (left image).(16-18)
As was usual in those days, diagnosis was way ahead of treatment for these disorders. Medical science had little to offer the RSD patient. If you can’t cure it, then the next best thing is to rename it. RSD has now been renamed “Complex Regional Pain Syndrome”.
Above left image: Bone scan showing increased activity in the left foot, indicating positive study for RSD, reflex sympathetic dystrophy, courtesy of medscape.com.
Successful Outcomes Using LDN
This is exactly why I found reports of successful outcomes using LDN for RDS so intriguing. RSD patients drag themselves from clinic to clinic having no effective treatment for the relentless progression of disease. They have little to offer except chronic narcotic addiction which is actually worse than the underlying disease.
Fibromyalgia Related to RSD
A number of studies and anecdotal reports show that LDN is useful for the fibromyalgia patient. (8-15) Perhaps Fibromyalgia and Chronic Regional Pain Syndrome are related in some way, since LDN seems to be effective for fibromyalgia as well. Perhaps the common thread is microglia activation in the central nervous system. LDN is an anti-inflammatory agent in the central nervous system, via action on microglial cells. (5)
According to Pradeep Chopra, MD ” In painful conditions such as Complex regional pain) and neuropathic pain, damage to the peripheral nerves shifts the glia to an activated state within the spinal cord….Glia are activated by trauma, injury, infection, opioids. When activated, glia release pro-inflammatory and neurotoxic factors (cytokines)….Drugs that block the effect of opioids (morphine) may help prevent activation of glia. Such drugs are naltrexone and naloxone. Low dose naltrexone (hence, LDN) may inhibit the activation of glia.”(6)
Nancy Sajben MD comments: ” New science shows naltrexone to be a potent anti-inflammatory — much stronger and with a much different mechanism than the weaker cox inhibitors such as ibuprofen, Vioxx, Celebrex, Naproxen with none of those adverse side effects. Dr. Hong reports that in animal studies, dextromethorphan is even stronger than naltrexone.”(20)
Chronic Major Depression – LDN Effective
Nancy Sajben MD suggests the anti-inflammatory activity of LDN explains its effectiveness in Major Depression refractory to the usual medications such as SSRI drugs. (19-20)
Medicinal Marijuana, Cannabis Sativa – Cannabinoids – Complex Regional Pain Syndrome
Another treatment modality showing success against RSD chronic refractory pain is medicinal marijuana.(21) “An oral cannabinoid was associated with up to 60% reductions in pain in 10 patients with refractory complex regional pain syndrome (CRPS), according to research presented at the 2010 annual World Congress on Pain in Montreal. The study investigators also reported that most of the patients were able to discontinue long-term opioid therapy and reported signi ficant improvements in quality of life.” (21) from Pharmacy Practice News. (More on this report)
Here is a review article on use of medicinal cannabis for allodynia, neuropathic pain and other chronic pain syndromes: Cannabinergic Pain Medicine Aggarwal_2013 Clinical Journal of Pain.
Cannabis for Fibromyalgia Pain – works better than prescription medications. “Medical marijuana is far more effective at treating symptoms of fibromyalgia than any of the three prescription drugs Cymbalta, Lyrica and Savella” (22)
Cannabis for Chronic Pain (23-25)
Current treatment of chronic pain leaved much to be desired. Chronic opiate use is not a good long term solution, yet is commonly employed. Studies show that in chronic patients on long term opiates, they report an augmented reduction in pain when vaporized cannabis is inhaled (25) Cannabis has been suggested as an adjunct or substitute for opiates in the treatment of chronic pain. This is our goal, to wean patients off addictive and dangerous opiates to safer alternatives. Any day a chronic pain patient can be weaned off opiates, that a good day.
A review of the literature showed 15 of 18 studies report analgesic effects of medical cannabis exceeding placebo, with improved sleep, and reporting no serious adverse effects.(23-25)
Medical cannabis is effective for chronic pain because it reduces perception of pain, is anti-inflammatory and affects voltage-gated sodium channels in nerves similar to action of neve blocking such such as lidocaine.
Dr Elizabeth Rahn from Athens Georia 2009 review is an excellent summary of animal and human research on cannabis for neuropathic pain.
Anecdotal Reports:
Below: Video testimony from Aamann Degarth using hemp oil for RSD , Nov 2011
Below: Part Two Video: from Aamann Degarth using hemp oil for RSD , June 2012
Elaine’s Story – 20 years of opiate refractory RSD, finally better with when Elaine tries medicinal cannabis for pain.
Also See the next article: Neuropathic Pain, Part Two
Articles with Related Interest:
Remission from Crohns Disease with Low Dose Naltrexone
Low Dose Naltrexone LDN Part One
Low Dose Naltrexone LDN Part Two
Low Dose Naltrexone LDN Part Three
Suppression of Research on Medical Cannabis
Jeffrey Dach MD
7450 Griffin Road, Suite 180/190
Davie, Fl 33314
954-792-4663
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Link to this article:http://wp.me/p3gFbV-1hZ
Links and references:
1) http://www.ncbi.nlm.nih.gov/
Am J Gastroenterol. 2007 Apr;102(4):820-8. Epub 2007 Jan 11.
Low-dose naltrexone therapy improves active Crohn’s disease.
Smith JP1, Stock H, Bingaman S, MauRger D, Rogosnitzky M, Zagon IS.
2) Dig Dis Sci. 2011 Jul;56(7):2088-97. doi: 10.1007/s10620-011-1653-7. Epub 2011 Mar 8. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial.
Smith JP1, Bingaman SI, Ruggiero F, Mauger DT, Mukherjee A, McGovern CO, Zagon IS.
3) http://www.ncbi.nlm.nih.gov/
Exp Biol Med (Maywood). 2009 Nov;234(11):1383-92. doi: 10.3181/0906-RM-189. Endogenous opioids regulate expression of experimental autoimmune encephalomyelitis: a new paradigm for the treatment of multiple sclerosis.
Zagon IS1, Rahn KA, Turel AP, McLaughlin PJ.
4) http://www.ncbi.nlm.nih.gov/
Ann Neurol. 2010 Aug;68(2):145-50. doi: 10.1002/ana.22006.
Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis.
Cree BA1, Kornyeyeva E, Goodin DS.
———————————————
5) http://www.ncbi.nlm.nih.gov/pubmed/24526250
Clin Rheumatol. 2014 Feb 15. [Epub ahead of print]
The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Younger J1, Parkitny L, McLain D.
Low-dose naltrexone (LDN) has been demonstrated to reduce symptom severity in conditions such as fibromyalgia, Crohn’s disease, multiple sclerosis, and complex regional pain syndrome. We review the evidence that LDN may operate as a novel anti-inflammatory agent in the central nervous system, via action on microglial cells. These effects may be unique to low dosages of naltrexone and appear to be entirely independent from naltrexone’s better-known activity on opioid receptors. As a daily oral therapy, LDN is inexpensive and well-tolerated. Despite initial promise of efficacy, the use of LDN for chronic disorders is still highly experimental. Published trials have low sample sizes, and few replications have been performed. We cover the typical usage of LDN in clinical trials, caveats to using the medication, and recommendations for future research and clinical work. LDN may represent one of the first glial cell modulators to be used for the management of chronic pain disorders.
—–
6) http://www.ldnresearchtrust.
Low Dose Naltrexone and chronic pain – Pradeep Chopra, MD
In painful conditions such as Complex regional pain) and neuropathic pain, damage to the peripheral nerves shifts the glia to an activated state within the spinal cord. Glia are activated by trauma, injury, infection, opioids. When activated, glia release pro-inflammatory and neurotoxic factors (cytokines).
Drugs that block the effect of opioids (morphine) may help prevent activation of glia. Such drugs are naltrexone and naloxone. Low dose naltrexone (hence, LDN) may inhibit the activation of glia.
It should not be taken by patients who are on opioids or tramadol.
7) http://www.ncbi.nlm.nih.gov/
J Neuroimmune Pharmacol. 2013 Jun;8(3):470-6. doi: 10.1007/s11481-013-9451-y. Epub 2013 Apr 2.
Treatment of Complex Regional Pain Syndrome (CRPS) using low dose naltrexone (LDN). Chopra P1, Cooper MS.
Fibromyalgia
8) http://www.ncbi.nlm.nih.gov/
Arthritis Rheum. 2013 Feb;65(2):529-38. doi: 10.1002/art.37734.
Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Younger J1, Noor N, McCue R, Mackey S.
To determine whether low dosages (4.5 mg/day) of naltrexone reduce fibromyalgia severity as compared with the nonspecific effects of placebo. In this replication and extension study of a previous clinical trial, we tested the impact of low-dose naltrexone on daily self-reported pain. Secondary outcomes included general satisfaction with life, positive mood, sleep quality, and fatigue.
METHODS:Thirty-one women with fibromyalgia participated in the randomized, double-blind, placebo-controlled, counterbalanced, crossover study. During the active drug phase, participants received 4.5 mg of oral naltrexone daily. An intensive longitudinal design was used to measure daily levels of pain.
RESULTS:When contrasting the condition end points, we observed a significantly greater reduction of baseline pain in those taking low-dose naltrexone than in those taking placebo (28.8% reduction versus 18.0% reduction; P = 0.016). Low-dose naltrexone was also associated with improved general satisfaction with life (P = 0.045) and with improved mood (P = 0.039), but not improved fatigue or sleep. Thirty-two percent of participants met the criteria for response (defined as a significant reduction in pain plus a significant reduction in either fatigue or sleep problems) during low-dose naltrexone therapy, as contrasted with an 11% response rate during placebo therapy (P = 0.05). Low-dose naltrexone was rated equally tolerable as placebo, and no serious side effects were reported.
CONCLUSION:The preliminary evidence continues to show that low-dose naltrexone has a specific and clinically beneficial impact on fibromyalgia pain. The medication is widely available, inexpensive, safe, and well-tolerated. Parallel-group randomized controlled trials are needed to fully determine the efficacy of the medication.
9)http://www.ncbi.nlm.nih.gov/
Pain Med. 2009 May-Jun;10(4):663-72. doi: 10.1111/j.1526-4637.2009.
Fibromyalgia is a chronic pain disorder that is characterized by diffuse musculoskeletal pain and sensitivity to mechanical stimulation. In this pilot clinical trial, we tested the effectiveness of low-dose naltrexone in treating the symptoms of fibromyalgia. Participants completed a single-blind, crossover trial with the following time line: baseline (2 weeks), placebo (2 weeks), drug (8 weeks), and washout (2 weeks).
PATIENTS:Ten women meeting criteria for fibromyalgia and not taking an opioid medication.
INTERVENTIONS:Naltrexone, in addition to antagonizing opioid receptors on neurons, also inhibits microglia activity in the central nervous system. At low doses (4.5 mg), naltrexone may inhibit the activity of microglia and reverse central and peripheral inflammation.
OUTCOME MEASURES:Participants completed reports of symptom severity everyday, using a handheld computer. In addition, participants visited the lab every 2 weeks for tests of mechanical, heat, and cold pain sensitivity.
RESULTS:Low-dose naltrexone reduced fibromyalgia symptoms in the entire cohort, with a greater than 30% reduction of symptoms over placebo. In addition, laboratory visits showed that mechanical and heat pain thresholds were improved by the drug. Side effects (including insomnia and vivid dreams) were rare, and described as minor and transient. Baseline erythrocyte sedimentation rate predicted over 80% of the variance in drug response. Individuals with higher sedimentation rates (indicating general inflammatory processes) had the greatest reduction of symptoms in response to low-dose naltrexone.
CONCLUSIONS:We conclude that low-dose naltrexone may be an effective, highly tolerable, and inexpensive treatment for fibromyalgia.
10)http://www.ncbi.nlm.nih.gov/
Psychosomatics. 2012 Nov-Dec;53(6):591-4. doi: 10.1016/j.psym.2011.11.006. Epub 2012 Apr 4. Is fibromyalgia an endocrine/endorphin deficit disorder? Is low dose naltrexone a new treatment option? Ramanathan S1, Panksepp J, Johnson B.
http://www.cortjohnson.org/
Low Dose Naltrexone (LDN) for Fibromyalgia and Chronic Fatigue Syndrome
12) http://med.stanford.edu/snapl/
Low-Dose Naltrexone Reduces the Symptoms of Fibromyalgia
Fibromyalgia Symptoms are reduced by low-dose naltrexone: A pilot study.
Pain Medicine (2009) Jarred W. Younger and Sean C. Mackey
13)http://chronicfatigue.about.
More Positive Results: Low-Dose Naltrexone for Fibromyalgia
By Adrienne DellwoFebruary 8, 201314)
14)http://www.amazon.com/forum/
Ms. Linda G. Zambanini says: I am an RN and Information Scientist who researches novel adjuvant cancer therapies and i had fibromyalgia. I say HAD because i no longer have fibromyalgia pain. I was in horrible pain for 8 years prior to that. I initially learned about LDN as a novel adjuvant for cancer – but soon learned it was also being used for fibromyalgia. I have been on Low Dose Naltrexone (also called LDN) for over a year now and the results have been nothing short of miraculous. It is an old, off-patent drug that is compounded and used in dosages less than a 1/10th of the original dosage. As such it functions as an immunomodulator. That is, if one’s immune system is hyperactive as with autoimmune diseases like SLE and MS it brings it down to normal; and if one’s immune system is hypoactive as with cancer and HIV it brings it up to normal. LDN is currently being being studied for fibromyalgia at Stanford; but only after getting funding from the Fibromyalgia Society since drug companies were not interested (in fact they are not interested in it for cancer or MS or any other indication) – it is an old off patent drug which one can buy for almost nothing. (I get 3 months worth from a compounding pharmacy in Colorado -Belmar Pharmacy – for about $40). From the research i had done prior to taking it most reported it took several months for it to work in the case of fibromyalgia (much faster results with MS). Sure enough it did not work for me initially, but knowing what to expect i continued it and after 2 months i became painfree! It had been so long since i had been pain free i had almost forgotten what that was like. I hope everyone with fibromyalgia looks into LDN. In fact if you know anyone with Cancer, SLE, Crohns disease, MS, HIV etc… please let them know about LDN. There are many videos on Youtube about LDN also. Just type LDN or low dose naltrexone in the search box.www.lowdosenaltrexone.org http://snapl.stanford.edu/ldn/
15) http://snapl.stanford.edu/
The Use of Low Dose Naltrexone in Fibromyalgia
Interview with Dr. Jarred Younger, Instructor in the Department of Anesthesia at Stanford University School of Medicine Topic: The Use of Low Dose Naltrexone in Fibromyalgia-
16)http://emedicine.medscape.com/
Reflex Sympathetic Dystrophy Imaging Lawrence E Holder, MD; Chief Editor: Felix S Chew, MD
17)http://www.ncbi.nlm.nih.gov/
Arch Phys Med Rehabil. 1989 Feb;70(2):135-7.
Predictive value of the three-phase technetium bone scan in diagnosis of reflex sympathetic dystrophy syndrome. Davidoff G1, Werner R, Cremer S, Jackson MD, Ventocilla C, Wolf L.
http://www.ncbi.nlm.nih.gov/
Indian J Nucl Med. 2013 Jan;28(1):11-6. doi: 10.4103/0972-3919.116798.
Usefulness of asymmetry score on quantitative three-phase bone scintigraphy in the evaluation of complex regional pain syndrome.
Sampath S1, Mittal BR, Arun S, Sood A, Bhattacharya A, Sharma A.
18)http://www.ncbi.nlm.nih.gov/
Clin J Pain. 2010 Mar-Apr;26(3):182-9. doi: 10.1097/AJP.0b013e3181c20207.
Sensitivity and specificity of 3-phase bone scintigraphy in the diagnosis of complex regional pain syndrome of the upper extremity.
Wüppenhorst N1, Maier C, Frettlöh J, Pennekamp W, Nicolas V.
Joint and bone alterations are seldom mentioned in the diagnostic criteria for complex regional pain syndrome (CRPS) even though they are important for long-term outcome. Altered periarticular bone metabolism can be detected by 3-phase bone scintigraphy (TPBS). Although frequently examining the diagnostic efficacy of TPBS is debatable.
METHODS:In all, 78 TPBS (45 CRPS/33 control group) were evaluated qualitatively and quantitatively. Sensitivity and specificity of the qualitative blinded reviewer analysis (n=57) compared with quantitative region of interest (ROI)-based analysis over the metacarpophalangeal, proximal, and distal interphalangeal joints (n=74) were evaluated. Patients’ sex, age, duration of CRPS, inciting event, extent of joint alteration, and handedness were included as covariables.
RESULTS:Qualitative blinded reviewer TPBS analysis had a high specificity (83%-100%). However, sensitivity was 31% to 50%. Interrater reliability was moderate (kappa score 0.56). Using the ROI-based evaluation, the highest sensitivity (69%) and specificity (75%) (ROI score > or =1.32) was shown for phase 3, whereas sensitivity of phases 1 and 2 rapidly declined to 50%. Duration of CRPS until TPBS was the only variable with significant impact on ROI scores of phase 3 (F=23.7; P=0.000; R=0.42). ROI scores declined with increasing duration of CRPS.
DISCUSSION:In conclusion, TPBS is a highly specific tool for diagnosing CRPS of the upper limb. ROI evaluation of phase 3 within the first 5 months after onset of CRPS is an appropriate additional diagnostic tool to confirm or exclude CRPS of the upper extremity.
Nancy Sajben MD
19)http://painsandiego.com/2014/
Low dose naltrexone (LDN), novel anti-inflammatory treatment for chronic pain
02/15/2014 — Nancy Sajben MD
20) http://painsandiego.com/2009/
Nancy Sajben MD Low Dose Naltrexone “LDN” and Dextromethorphan off label for Pain, RSD, Chronic Fatigue, Fibromyalgia, MS, Crohn’s Disease
05/26/2009 — Nancy Sajben MD
New science shows naltrexone to be a potent anti-inflammatory — much stronger and with a much different mechanism than the weaker cox inhibitors such as ibuprofen, Vioxx, Celebrex, Naproxen with none of those adverse side effects. Dr. Hong reports that in animal studies, dextromethorphan is even stronger than naltrexone.
(21) http://www.braatah.com/
Refractory CRPS Patients Discontinue Opiates With Cannabinoid Treatment by David Wild, Pharmacy Practice News
Montreal—An oral cannabinoid was associated with up to 60% reductions in pain in 10 patients with refractory complex regional pain syndrome (CRPS), according to research presented at the 2010 annual World Congress on Pain in Montreal. The study investigators also reported that most of the patients were able to discontinue long-term opioid therapy and reported significant improvements in quality of life.
Marijuana Rated Most Effective for Treating Fibromyalgia
April 21st, 2014 by Pat Anson, Editor
Medical marijuana is far more effective at treating symptoms of fibromyalgia than any of the three prescription drugs approved by the Food and Drug Administration to treat the disorder.
That is one of the surprise findings in an online survey of over 1,300 fibromyalgia patients conducted by the National Pain Foundation and National Pain Report.
Cymbalta graphThe FDA has approved only three drugs – Cymbalta, Lyrica and Savella — for the treatment of fibromyalgia. Although they generate billions of dollars in annual sales for Pfizer, Eli Lilly, Forest Laboratories and other drug makers, most who have tried the medications say they don’t work.
——————————————————
23) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3939704/
Front Pharmacol. 2014 Mar 3;5:28. full text free
Targeting the endogenous cannabinoid system to treat neuropathic pain. Lau BK, Vaughan CW. Pain Management Research Institute, Kolling Institute of Medical Research, Northern Clinical School, University of Sydney Sydney, NSW, Australia.
the currently recommended pharmacological treatments for neuropathic pain display poor efficacy and illicit undesirable side effects
free full text
24) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3243008/
Lynch M. E., Campbell F. (2011). Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials.
Br J Clin Pharmacol. Nov 2011; 72(5): 735–744.
Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials
Mary E Lynch1 and Fiona Campbell2 1Department Anesthesia, Psychiatry, Dalhousie University, Halifax, Canada
2Department of Anaesthesia and Pain Medicine, Hospital for Sick Children, University of Toronto, Toronto, Canada
Dr Mary E. Lynch, MD, FRCPC, Pain Management Unit, Queen Elizabeth II Health Sciences Centre, 4thFloor Dickson Centre, Room 4086, Halifax, Nova Scotia,
Fifteen of the eighteen trials that met the inclusion criteria demonstrated a significant analgesic effect of cannabinoid as compared with placebo and several reported significant improvements in sleep. There were no serious adverse effects. Adverse effects most commonly reported were generally well tolerated, mild to moderate in severity and led to withdrawal from the studies in only a few cases. Overall there is evidence that cannabinoids are safe and modestly effective in neuropathic pain with preliminary evidence of efficacy in fibromyalgia and rheumatoid arthritis.
25) http://www.ncbi.nlm.nih.gov/pubmed/22048225
Clin Pharmacol Ther. 2011 Dec;90(6):844-51. doi: 10.1038/clpt.2011.188. Epub 2011 Nov 2.
Cannabinoid-opioid interaction in chronic pain.
Abrams DI1, Couey P, Shade SB, Kelly ME, Benowitz NL.
Cannabinoids and opioids share several pharmacologic properties and may act synergistically. The potential pharmacokinetics and the safety of the combination in humans are unknown. We therefore undertook a study to answer these questions. Twenty-one individuals with chronic pain, on a regimen of twice-daily doses of sustained-release morphine or oxycodone were enrolled in the study and admitted for a 5-day inpatient stay. Participants were asked to inhale vaporized cannabis in the evening of day 1, three times a day on days 2-4, and in the morning of day 5. Blood sampling was performed at 12-h intervals on days 1 and 5. The extent of chronic pain was also assessed daily. Pharmacokinetic investigations revealed no significant change in the area under the plasma concentration-time curves for either morphine or oxycodone after exposure to cannabis. Pain was significantly decreased (average 27%, 95% confidence interval (CI) 9, 46) after the addition of vaporized cannabis. We therefore concluded that vaporized cannabis augments the analgesic effects of opioids without significantly altering plasma opioid levels. The combination may allow for opioid treatment at lower doses with fewer side effects.
_________________
Russo EB
http://www.ncbi.nlm.nih.gov/pubmed/18728714
Ther Clin Risk Manag. 2008 Feb;4(1):245-59.
Cannabinoids in the management of difficult to treat pain.
Russo EB. GW Pharmaceuticals Vashon, WA, USA.
This article reviews recent research on cannabinoid analgesia via the endocannabinoid system and non-receptor mechanisms, as well as randomized clinical trials employing cannabinoids in pain treatment. Tetrahydrocannabinol (THC, Marinol((R))) and nabilone (Cesamet((R))) are currently approved in the United States and other countries, but not for pain indications. Other synthetic cannabinoids, such as ajulemic acid, are in development. Crude herbal cannabis remains illegal in most jurisdictions but is also under investigation. Sativex((R)), a cannabis derived oromucosal spray containing equal proportions of THC (partial CB(1) receptor agonist ) and cannabidiol (CBD, a non-euphoriant, anti-inflammatory analgesic with CB(1) receptor antagonist and endocannabinoid modulating effects) was approved in Canada in 2005 for treatment of central neuropathic pain in multiple sclerosis, and in 2007 for intractable cancer pain. Numerous randomized clinical trials have demonstrated safety and efficacy for Sativex in central and peripheral neuropathic pain, rheumatoid arthritis and cancer pain. An Investigational New Drug application to conduct advanced clinical trials for cancer pain was approved by the US FDA in January 2006. Cannabinoid analgesics have generally been well tolerated in clinical trials with acceptable adverse event profiles. Their adjunctive addition to the pharmacological armamentarium for treatment of pain shows great promise.
excellent review full text
http://europepmc.org/articles/PMC2755639
Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside Rahn Elizabeth J. (1) ; Hohmann Andrea G. (1) ;
Neuroscience and Behavior Program, Department of Psychology, University of Georgia, 30602-3013, Athens,
Neuropathic pain is a debilitating form of chronic pain resulting from nerve injury, disease states, or toxic insults. Neuropathic pain is often refractory to conventional pharmacotherapies, necessitating validation of novel analgesics. Cannabinoids, drugs that share the same target as Δ9-tetrahydrocannabinol (Δ9-THC), the psychoactive ingredient in cannabis, have the potential to address this unmet need. Here, we review studies evaluating cannabinoids for neuropathic pain management in the clinical and preclinical literature. Neuropathic pain associated with nerve injury, diabetes, chemotherapeutic treatment, human immunodeficiency virus, multiple sclerosis, and herpes zoster infection is considered. In animals, cannabinoids attenuate neuropathic nociception produced by traumatic nerve injury, disease, and toxic insults. Effects of mixed cannabinoid CB1/CB2 agonists, CB2 selective agonists, and modulators of the endocannabinoid system (i.e., inhibitors of transport or degradation) are compared. Effects of genetic disruption of cannabinoid receptors or enzymes controlling endocannabinoid degradation on neuropathic nociception are described. Specific forms of allodynia and hyperalgesia modulated by cannabinoids are also considered. In humans, effects of smoked marijuana, synthetic Δ9-THC analogs (e.g., Marinol, Cesamet) and medicinal cannabis preparations containing both Δ9-THC and cannabidiol (e.g., Sativex, Cannador) in neuropathic pain states are reviewed. Clinical studies largely affirm that neuropathic pain patients derive benefits from cannabinoid treatment. Subjective (i.e., rating scales) and objective (i.e., stimulus-evoked) measures of pain and quality of life are considered. Finally, limitations of cannabinoid pharmacotherapies are discussed together with directions for future research.
http://www.lowdosenaltrexone.
Jeffrey Dach MD
7450 Griffin Road, Suite 180/190
Davie, Fl 33314
954-792-4663
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/disc.yourwebapps.com/Indices/244124.html
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
disc.yourwebapps.com/Indices/244161.html
disc.yourwebapps.com/Indices/244163.html
Disclaimer click here: www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article:http://wp.me/p3gFbV-1hZ
Copyright (c) 2014 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.









Medical Cannabis States Have Less Mortality From Opiates - Jeffrey Dach MD December 24, 2014 at 9:03 AM
[…] LDN Part Four […]