by Jeffrey Dach MD
Lately, we have seen a growing number of young women using the NuvaRing, reporting anxiety, depression, insomnia, headache, and other symptoms. The NuvaRing is a contraceptive device (IUD) containing synthetic hormones, a form of birth control.
Above Left Image: NuvaRing Contraceptive Device . This device is impregnated with synthetic hormones that cause blood clots and stroke. Courtesy of Wikimedia Commons.
My previous article discussed the adverse effects of synthetic hormones in birth control pills.(1) One dreaded complication is embolic stroke. Working in the hospital as an interventional radiologist over the years, one of my jobs was to perform angiograms on young women who had strokes while on birth control pills. The referring doctors ordered angiograms on these young women to get a look at the cerebral arteries after the fact.
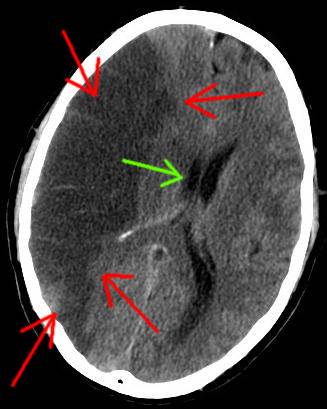 Left Image : CAT Scan of right middle cerebral artery stroke, (red arrows) . This is a cerebrovascular accident due to occlusion of middle cerebral artery. Green arrow denotes midline shift. This patient was left with a permanent paralysis of the left arm and leg. Courtesy of wikimedia commons.
Left Image : CAT Scan of right middle cerebral artery stroke, (red arrows) . This is a cerebrovascular accident due to occlusion of middle cerebral artery. Green arrow denotes midline shift. This patient was left with a permanent paralysis of the left arm and leg. Courtesy of wikimedia commons.
These unfortunate victims had already recovered from the arterial occlusion and most had recovered function. However, some had completed strokes with permanent neurological damage with weakness or paralysis of an upper of lower extremity.(see above image of CAT scan)
Seeing these young women with residual stroke created an incongruous image in my mind which I cannot forget, the image of an otherwise youthful and healthy young women, a mother or college student, holding an debilitated, paralyzed arm at her side. After seeing a number of these victims, I must admit I developed an unease and discomfort with a mainstream medical system that creates such victims as an acceptable risk of doing business.
The older birth control pill has been supplanted by the newer Nuva-Ring which is another method to deliver synthetic hormones into the female body to prevent pregnancy. The Nuva-Ring increases the risk of blood clot formation, stroke and heart attack. A recent 2012 NEJM study which showed a 300% increased risk of stroke and heart attack in Nuva Ring users compared to placebo.(2) This is not good, and such negative findings are usually the death knell for the drug which ends up banned or at least a Black Box warning. None of this has happened yet for the Nuva Ring.
My previous article dealt with warning signs that let you know you are dealing with a bad drug. One of these is to take a look at the FDA adverse reporting system, to see numbers and types of adverse events. (3,4,5) (8)
As of Sep 3, 2012: 8,418 people reported side effects from the Nuvaring.(5)
(1) Deep vein thrombosis : 3,434 reports.
(2) Pulmonary embolism : 2,467 reports.
(3) Anxiety : 1,387 reports. and is moderate
(4) Metrorrhagia : 1,253 reports.
(5) Headache : 829 reports.
(6) Depression: 652 reports.
(7) Nausea : 646 reports.
(8) Migraine: 633 reports. It’s more common among females aged 20-29 years old, and can be severe
(9) Thrombosis : 549 reports.
(10) Hypercoagulation : 483 reports.
(11) insomnia
Another warning sign you might be dealing with a “bad drug” is the number of people with legal cases in drug litigation. (6,7) There are about a thousand women claiming in court that the Nuva-Ring caused their stroke, heart attack or death. (6,7) This is not a good sign.
Update Jan 2014: Article in Vanity Fair
In Vanity Fair Magazine: Danger in the Ring By Marie Brenner
“When 24-year-old Erika Langhart—talented, beautiful, bound for law school—died on Thanksgiving Day 2011, she became one of thousands of suspected victims of the birth-control device NuvaRing. Elite army athlete Megan Henry, who survived rampant blood clots in her 20s, is another. With major suits against NuvaRing’s manufacturer, Merck, headed for trial, Marie Brenner asks why, despite evidence of serious risk, a potentially lethal contraceptive remains on the market.” excerpt from article
For women who want some form of birth control to avoid pregnancy, a healthier non-hormonal option is the Copper T- IUD from Paragard. This is now recommended by the American College of OB/Gyn for women of all child bearing ages. The Copper T IUD is safer and more effective, and less costly than the traditional Oral Contraceptive Pill, as was discussed in my previous article. The Copper T- IUD is vastly safer than the Nuva-Ring, since the Copper T – IUD is Non-Hormonal, meaning it does not contain the synthetic hormones that cause stroke, heart attack and death.
The blood clotting effects of synthetic hormones pills are well known. However, the use of transdermal bioidentical estrogen is safe and not associated with blood clot formation. These medical studies are discussed in my previous two articles listed here:
The Safety of Transdermal Estrogen Part one
The Safety of Transdermal Estrogen Part Two
Articles with Related Interest:
Adverse Effects of Birth Control Pills
Protect Your Family From Bad Drugs
Author/: Jeffrey Dach MD
Links and References
1) jeffreydach.com/2011/08/24/adverse_effects_bcps_birth-control_pills_jeffrey_dach.aspx
ADverse effects of Birth Control Pills Nuvaring Stroke and MI
2) www.nejm.org/doi/full/10.1056/NEJMoa1111840?query=featured_home
N Engl J Med. 2012 Jun 14;366(24):2257-66.
Thrombotic stroke and myocardial infarction with hormonal contraception. Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Source Gynecologic Clinic 4232, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
BACKGROUND:Although several studies have assessed the risk of venous thromboembolism with newer hormonal contraception, few have examined thrombotic stroke and myocardial infarction, and results have been conflicting. METHODS: In this 15-year Danish historical cohort study, we followed nonpregnant women, 15 to 49 years old, with no history of cardiovascular disease or cancer. Data on use of hormonal contraception, clinical end points, and potential confounders were obtained from four national registries.
RESULTS: A total of 1,626,158 women contributed 14,251,063 person-years of observation, during which 3311 thrombotic strokes (21.4 per 100,000 person-years) and 1725 myocardial infarctions (10.1 per 100,000 person-years) occurred. As compared with nonuse, current use of oral contraceptives that included ethinyl estradiol at a dose of 30 to 40 μg was associated with the following relative risks (and 95% confidence intervals) for thrombotic stroke and myocardial infarction, according to progestin type: norethindrone, 2.2 (1.5 to 3.2) and 2.3 (1.3 to 3.9); levonorgestrel, 1.7 (1.4 to 2.0) and 2.0 (1.6 to 2.5); norgestimate, 1.5 (1.2 to 1.9) and 1.3 (0.9 to 1.9); desogestrel, 2.2 (1.8 to 2.7) and 2.1 (1.5 to 2.8); gestodene, 1.8 (1.6 to 2.0) and 1.9 (1.6 to 2.3); and drospirenone, 1.6 (1.2 to 2.2) and 1.7 (1.0 to 2.6), respectively. With ethinyl estradiol at a dose of 20 μg, the corresponding relative risks according to progestin type were as follows: desogestrel, 1.5 (1.3 to 1.9) and 1.6 (1.1 to 2.1); gestodene, 1.7 (1.4 to 2.1) and 1.2 (0.8 to 1.9); and drospirenone, 0.9 (0.2 to 3.5) and 0.0. For transdermal patches, the corresponding relative risks were 3.2 (0.8 to 12.6) and 0.0, and for a vaginal ring, 2.5 (1.4 to 4.4) and 2.1 (0.7 to 6.5).
CONCLUSIONS: Although the absolute risks of thrombotic stroke and myocardial infarction associated with the use of hormonal contraception were low, the risk was increased by a factor of 0.9 to 1.7 with oral contraceptives that included ethinyl estradiol at a dose of 20 μg and by a factor of 1.3 to 2.3 with those that included ethinyl estradiol at a dose of 30 to 40 μg, with relatively small differences in risk according to progestin type. (Funded by the Danish Heart Association.).
Adverse Reporting System FDA
3) www.druginformer.com/search/side_effect/nuvaring
NuvaRing- 32,039 events reported to the FDA adverse drug reporting system
4) www.fda-reports.com/nuvaring/reaction/insomnia/page2.html
nuvaring- 91 fda reports
5) www.ehealthme.com/ds/
Nuvaring side effects and drug interactions. – 8,418 reports from FDA and user community.
What is Nuvaring Nuvaring has active ingredients of ethinyl estradiol; etonogestrel. It is used in birth control, over-the-counter birth control, endometriosis, polycystic ovary disease, perimenopause.
On Sep, 3, 2012: 8,418 people who reported to have side effects when taking Nuvaring are studied
Trend of Nuvaring’s drug interactions, side effects, and effectiveness reports If people were to have side effects while taking Nuvaring, what are they:
(1) Deep vein thrombosis (?): 3,434 reports.
(2) Pulmonary embolism (?):
2,467 reports.
(3) Anxiety (?): 1,387 reports. and is moderate
(4) Metrorrhagia (?): 1,253 reports.
(5) Headache (?): 829 reports.
(6) Depression (?): 652 reports.
(7) Nausea (?): 646 reports.
(8) Migraine (?): 633 reports. It’s more common among females aged 20-29 years old, and can be severe
(9) Thrombosis (?): 549 reports.
(10) Hypercoagulation (?): 483 reports.
(11) insomnia
NuvaRing Lawsuits
6) news.yahoo.com/study-finds-increased-risk-nuvaring-stroke-heart-attack-002618487.html
New Study Finds Increased Risk Of NuvaRing Stroke and Heart Attack Merck & Co., the manufacturer of NuvaRing, is currently defending over 950 NuvaRing lawsuits. Plaintiffs in the lawsuits allege that the company sold a defectively designed product and failed to adequately warn users of the increased risk of NuvaRing adverse effects, such as stroke and heart attack. The majority of these cases are pending in a federal multidistrict litigation in the U.S. District Court for the Eastern District of Missouri before Judge Rodney Sippel (MDL No. 1964), and in a coordinated proceeding in Bergen County Superior Court of New Jersey before Judge Brian R. Martinotti (Docket No. BER-L-3081-09).
7) www.prweb.com/releases/prwebnuvaring-claim/nuvaring-lawsuits/prweb9821653.htm Experts Warn of NuvaRing Blood Clot Side Effects The NuvaRing Resource Center is the Web’s largest source for information on NuvaRing side effects, research and legal news. Visit www.NuvaRing-Lawsuits.net
PRWEB/// August 22, 2012
The NuvaRing Resource Center, a patient advocacy group, is alerting women who used the contraceptive NuvaRing that the device has been linked to increased risks of blood clots and related injuries. Lawyers are currently helping those affected determine their legal options. Anyone who suffered a blood clot, DVT, stroke or Pulmonary Embolism after using NuvaRing is urged to contact the NuvaRing Resource Center or speak with a lawyer about their legal options.
NuvaRing is a once-a-month vaginal ring contraceptive which Merck marketed as containing lower doses of hormones. However, the device releases chemicals directly into the bloodstream on a constant basis.
So far, the FDA has received over 1,000 reports of blood clot injury or death in patients using NuvaRing.
On October 27, 2011 they released a report titled, “Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints”, which showed vaginal ring contraceptives could increase the risks of blood clots by as much as 56% over traditional birth control pills.
The British Medical Journal published a study from Denmark on May 10, 2012 linking vaginal rings like NuvaRing to as much as a 90% increased risk of blood clots over oral contraceptives.
On June 14, 2012, the New England Journal of Medicine also published a Danish study finding vaginal ring contraceptives could relate to a 2.5 to 3-fold increased risk of blood clots. Due to the large number of NuvaRing lawsuits filed by patients, the cases have been consolidated into a federal Multi-District Litigation court in Missouri. The formal case is known as In re: NuvaRing Products Liability Litigation, No. 08-md-1964, JPML, Eastern District Missouri. So far, nearly 1,000 victims have filed a NuvaRing claim. The NuvaRing Resource Center urges anyone affected by blood clots, stroke, DVT or pulmonary embolism after using NuvaRing to learn their legal rights as soon as possible. Victims should make sure they choose a lawyer with experience in defective drug litigation. The Resource Center only recommends lawyers and law firms who have already handled NuvaRing lawsuits.
8) www.fda.gov/downloads/Drugs/DrugSafety/UCM277384.pdf
Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints FDA Office of Surveillance and Epidemiology, Lead Site Rita Ouellet-Hellstrom, Ph.D., M.P.H., FDA Principal Investigator David J. Graham, M.D., M.P.H., FDA Senior Science Advisor Judy A. Staffa, Ph.D., R.Ph., FDA Project Officer
More references:
1. Cardiovascular disease and steroid hormone contraception. Technical Report Series: WHO1998
2. Chan WS, Ray J, Wai EK, Ginsburg S, Hannah ME, Corey PN, Ginsberg JS.
Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence. Arch Intern Med. 2004 Apr 12;164(7):741-7
3. Baillargeon JP, McClish DK, Essah PA, Nestler JE.
Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: a metaanalysis. J Clin Endocrinol Metab. 2005 Jul;90(7):3863-70. Epub 2005 Apr 6.
4. Dinger JC, Heinemann LA, Kühl-Habich D.
The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007 May;75(5):344-54. Epub 2007 Feb 23
5. Dinger J, Assmann A, Möhner S, Minh TD.
Risk of venous thromboembolism and the use of dienogest- and drospirenone-containing oral contraceptives: results from a German case-control study. J Fam Plann Reprod Health Care. 2010 Jul;36(3):123-9.
6. Seeger JD, Loughlin J, Eng PM, Clifford CR, Cutone J, Walker AM.
Risk of thromboembolism in women taking ethinylestradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007 Sep;110(3):587-93.
7. Lidegaard Ø, Løkkegaard E, Svendsen AL, Agger C.
Hormonal contraception and risk of venous thromboembolism: national follow-up study.
BMJ. 2009 Aug 13;339:2890. doi: 10.1136/bmj.b28
8. van Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR.
The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. B
MJ. 2009 Aug 13;339:b2921. doi: 10.1136/bmj.b2921.
9. Parkin L, Sharples K, Hernandez RK, Jick SS.
Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ. 2011 Apr 21;342:d2139. doi: 10.1136/bmj.d2139.
10. Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ. 2011 Apr 21;342:d2151.
11. Jick SS, Kaye JA, Russmann S,
Jick H. Risk of non-fatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 µg if ethinyl estradiol. Contraception. 2006 73: 223-228.
12. Jick S, Kaye JA, Li L, Jick H.
Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 micrograms of ethinyl estradiol. Contraception. 2007 Jul;76(1):4-7. Epub 2007 May 11.
13. Jick SS, Hagberg KW, Kaye JA.
ORTHO EVRA and venous thromboembolism: an update. Contraception. 2010 May;81(5):452-3. Epub 2010 Jan 27. No abstract available.
14. Jick SS, Hagberg KW, Hernandez RK, Kaye JA.
Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception. 2010 Jan;81(1):16-21. Epub.
15. Jick SS, Jick H.
The contraceptive patch in relation to ischemic stroke and acute myocardial infarction.
Pharmacotherapy. 2007 Feb;27(2):218-20.
16. Cole JA, Norman H, Doherty M, Walker AM.
Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users.
Obstetrics and Gynecologist . 2007 Feb;109(2):339-346.
17. Dore DD, Norman H, Loughlin J, Seeger JD.
Extended case-control study results on thromboembolic outcomes among transdermal contraceptive users.
Contraception. 2010 May;81(5):408-13. Epub 2010 Jan 22.
18. Heinemann LAJ, Dinger JC.
Range of published estimates of venous thromboembolism incidence in young women.
Contraception 75:328-336, 2007.
19. Petitti DB, Sidney S, Quesenberry CP Jr, Bernstein A.
Incidence of stroke and myocardial infarction in women of reproductive age.
Stroke. 1997 Feb;28(2):280-3.
20. Ducros E, Berthaut A, Mirshahi SS, Faussat AM, Soria J, Agarwal MK, Mirshahi M.
Aldosterone modifies hemostasis via upregulation of the protein-C receptor in human vascular endothelium. Biochem Biophys Res Commun. 2008 Aug 22;373(2):192-6 33
21 ) http://www.huffingtonpost.com/2013/12/18/nuvaring-blood-clots_n_4461429.html
Side Effects May Include Death: The Story Of The Biggest Advance In Birth Control Since The Pill Sabrina Siddiqui
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244124.html
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
disc.yourwebapps.com/Indices/244161.html
disc.yourwebapps.com/Indices/244163.html
Disclaimer click here: www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article: http://wp.me/P3gFbV-Qr
Copyright (c) 2012-2013 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Holly October 23, 2013 at 10:29 AM
The nuvaring isn’t an intrauterine device at all, is it? It is just inserted into the vagina by the user and not the uterus by a medical professional. Have you published anything on mirena IUD?
Jeffrey Dach MD October 24, 2013 at 4:05 PM
Hi Holly,
Yes, you are quite right, about that. The Nuva-Ring is inserted by the user and remains below the cervix in the vaginal canal.
No visit to the Gyne office is needed as the user may remove the Nuva Ring on their own.
The Mirena also contains synthetic hormones and is not recommended as it causes a host of health problems including hypercoagulation, blood clot, stroke and disturbance of B vitamin metabolism. Most of the adverse effects of synthetic hormones from BCPs (birth control pills) are described in the article above. These also apply to the Mirena IUD. A safer choice is the Copper T IUD which is non-hormonal.
regards
jeffrey dach md
Karen Langhart January 16, 2014 at 7:21 AM
From: Karen
Date: Thu, Dec 19, 2013 at 2:13 PM
Subject: Thank you and our daughter Erika
Dear Dr. Dach,
We have been sharing your article: “Adverse Effects of Birth Control Pills” with many, many family and friends for over two years now, beginning with providing it to the hundreds of women in attendance of our daughter’s celebration of life in December of 2011. Thank you for your care and for your research to help women understand the serious dangers of synthetic hormones. We wish we had been aware of your excellent and comprehensive article sooner…below is our daughter’s story.
Our beautiful, caring, sweet 24 year old daughter, Erika, was planning to come home for Thanksgiving on Wednesday, November 23rd, 2011. On Monday, November 21st having just talked to her over the weekend and emailing her during that day about how much we (my husband and I) were looking forward to having her home for Thanksgiving from where she lived and worked in Washington DC; I received a call on my phone that evening from her phone. I picked up the call, expecting it to be her and said “Can’t wait to see you!!!”. Instead it was her boyfriend who told me that Erika had collapsed and was being rushed to the hospital. WHAT? HOW CAN THIS BE? He did not know. Frantically we contacted the emergency room where she was taken and talked with the ER doctor treating Erika. We learned that she had suffered, massive, double pulmonary embolisms, literally, out of nowhere. One of the very first questions the doctor asked was whether she or not she was on birth control, and if so what type. As soon as the doctors learned that she was using the Nuvaring, they removed it. They told us that we needed to get to the hospital as soon as we could. With just a couple of hours to prepare, we quickly did research on the Nuvaring relative to pulmonary embolisms and were shocked to find that there was a great deal of concern and many incidents of women suffering from blood clots that could then cause embolisms. It was even, currently, being studied by the FDA we learned.
We were able to get on a flight from Phoenix to Washington DC at midnight that night. With information printed out on the subject, we spent much of the flight reading about the serious issues of blood clots and pulmonary embolisms as a result of the use of the Nuvaring. To say we were terrified for our daughter is an understatement.
We arrived in Washington DC early Tuesday morning and went directly to the hospital. Erika was on life support and in a coma. We spent three torturous days hoping for the best and fearing the worst. The doctors told us that the pulmonary embolisms were a direct result of the Nuvaring. On Thanksgiving morning the neurosurgeon informed us that our sweet daughter Erika was irreversibly brain dead. That Thanksgiving day we chose, as we knew our caring, wonderful daughter would want us to, to donate her organs – all of which, with the exception of her lungs which were so terribly damaged that they could not be used, ultimately helped other people.
Two weeks after our daughter’s passing we were back in Washington DC to speak at the hearings that were ironically and sadly being conducted on these third and fourth generation contraceptives. Our only remaining purpose in life is to try to help Erika’s life make a difference.
So how to help make a difference? We obviously want women to be made aware of the dangers of the NuvaRing (article about our daughter and the NuvaRing was published this month in Vanity Fair magazine and yesterday in the Huffington Post
but we believe, as you have pointed out, the issue is much larger than this. We believe that women should be made aware that ALL synthetic hormones are dangerous, in so many ways, to their health. We do not understand why these drugs have been allowed to be on the market for 50 years – 50 years of damaging women’s health, when there is a safer, more cost effective and more effective method available – the Copper IUD. If the FDA is serious about their mission statement:
FDA’s Mission Statement
The FDA is responsible for protecting the public health by assuring the safety, efficacy and security of human and veterinary drugs, biological products, medical devices, our nation’s food supply, cosmetics and products that emit radiation. The FDA is also responsible for advancing the public health by helping to speed innovations that make medicines and foods more effective, safer, and more affordable; and helping the public get the accurate, science-based information they need to use medicines and foods to improve their health.
…then they would be advocating the use of the Copper IUD first and foremost as the method of choice for contraception. This brings us to our questions, which we would greatly appreciate your opinion on.
* Are there serious risks with the use of the Copper IUD? Are these risks preventable (controllable – ie if there are risks; are there early signs that an informed woman could be made aware of to avoid that risk?)
* If there are serious risks, how do they compare to the serious risks of hormonal birth control? How confidently can the Copper IUD be promoted as the safest form of birth control?
* Why is the cost of the Paragard IUD in the neighborhood of $700 in the US vs. $70 in Canada and even less in the rest of the world? When we attended the ACOG conference in San Diego in 2012 and asked this question we were told because of all of the R&D the US does…nonsense in our opinion. Furthermore, when we asked doctors why they do not recommend the Paragard IUD to their patients, they responded because it is “not reimbursable” by the insurance companies. Do you know what this means?
In our view, all of these tactics appear to be artificial ways to keep women from having access to the safest, most effective and least expensive form of birth control available – the copper IUD. We surmise that it is simply about money. If women could be using a safe and effective form of birth control that does not cause further health issues like hormonal birth control does (which then requires more costly doctor and pharmaceutical drug care), and one that should be at a cost of approximately $7 per year ($70/10 years); vs. an average $500 per year for hormonal birth control; then the medical and drug industry would be losing billions of dollars in profits each year -it is as simple, and as complicated, as that in our opinion. We believe that this is the real “War on Women”. Women simply have no “choice” if they are not receiving an INFORMED “choice”.
In order to do everything we can to help create an awareness on this issue; we hope to understand as much as we can about the Copper IUD – good and bad – in order to promote it as the best alternative for birth control in the US. We are very hopeful that you can help us with accessing this knowledge and information.
It has been very difficult to put all of this into words, so our apologies for the long winded email. If you would be willing to discuss this with us; in the interest of advancing the good health of millions of women in the U.S; we would be profoundly grateful.
Thank you, again, Dr. Dach for your dedication to this issue; and for what you have already accomplished in the interest of women’s health.
With Warm Regards,
Richard and Karen
DW March 4, 2014 at 3:05 AM
Dear Dr. Dach: Thank you for the post. My 29-year-old daughter died while she was on NuvaRing from blood clots in her lungs last year. She had been married for just 5 weeks when she became became so ill she had to go to the ER. They took the NuvaRing out, but nothing the doctors did could help and she died within 24 hours of being in the hospital.
The warnings on these prescription inserts and packages to call your doctor or go the hospital are not enough.
The FDA has accepted studies that show the least risk to women, instead of the studies that show there is a higher risk. Why they choose to ignore the higher risks and to allow Merck to minimize the true risk is beyond me. I do not trust the FDA as they are not protecting women.
I also do not understand why the medical community and others continue to compare blood clots for women who are pregnant against women who are trying not to get pregnant. One would think that people could understand that women who use birth control are trying not to get pregnant. So why not inform women about the safest birth control?
Nothing will ever replace the loss of my daughter, and I hope other young women will pay attention to the fact that all hormonal birth control has risk and that third and fourth generation progestins have a higher risk.
Thank you again for writing about this important issue.
Sincerely,
Dru
Adverse Effects of BCPs Birth Control Pills May 20, 2014 at 7:05 AM
[…] See the article: Bad News for the Nuva Ring […]
Jeffrey Dach MD Bad News on the NuvaRing by Jeffrey Dach MD - Jeffrey Dach MD April 7, 2015 at 4:50 PM
[…] Bad News for the NuvaRing by Jeffrey Dach MD Lately, we have seen a growing number of young women using the NuvaRing, reporting anxiety, depression, insomnia, headache, and other symptoms. The NuvaRing is a contraceptive intra-uterine device (IUD) containing synthetic hormones, a form of birth control…For More Click Here. […]