Challenge, Re-challenge Evidence for Hydroxychloroquine Efficacy Against Corona Virus
by Jeffrey Dach MD
Although the Randomized Double Blind Placebo Controlled Trial is considered the gold standard for FDA drug approval, this type of drug trial is not practical for off label drugs and natural substances which can not be patented. The reason patent protection is required is the cost involved in a randomized trial is prohibitively expensive in the range of 100 to 250 million dollars. No drug company in their right mind would incur this expense without patent protection to guarantee profits on the back end.
The Privilege of Off-Label Prescribing
About twenty percent (one fifth) of all drug prescriptions are prescribed Off Label.(1) This means the drug is prescribed for a different indication unrelated to the original FDA approved indication. An example of “Off-Label” use is the prescribing of Hydroxychloroquine (Plaquenil) for systemic lupus erythematosis (SLE). The drug was originally FDA approved for treatment of malaria which has nothing to do with systemic lupus. This common medical practice of “Off-Label Use” of a drug has always been accepted by all medical societies and regulatory agencies as a prerogative and privilege of the prescribing physician.
Since twenty percent of prescriptions are not based on randomized double blind placebo controlled trials typically used for FDA new drug approval, the next obvious question is: What other types evidence are used ?
Other Types of Evidence for Prescribing Drugs Off Label
Doctors rely on many other types of evidence such as in-vitro and in-vivo animal studies, human observational trials, registry trials, and challenge, rechallenge studies. Of course, the doctor must understand the basic science studies showing the mechanism of action of the drug. The importance of this cannot be over-emphasized, as it provides confidence that the drug is effective and can be used, or ineffective and should not be used.
The Sad Story of Procrit
For example, if basic science shows that a drug stimulates cancer cell growth, then obviously, this drug would not be suitable for the oncology patient. Such was the case with Procrit, a form of erythropoeitin manufactured with the new recombinant DNA technology. The drug was FDA approved to increase (RBC) Red Blood Cell count in anemic patients. Anemia is a common side effect of chemotherapy, and the drug was used for many years on oncology wards to increase blood counts in cancer patients. This was carried out for many years until it was finally discovered the drug increased the mortality from cancer by stimulating cancer growth. Basic science studies showed that many cancer cell types have erythropoetin receptors that stimulate the cancer cell with growth signals.(3-4)
Drug Challenge Rechallenge
Another form of evidence which proves drug causality accepted by medical science as well as our legal system (a court of law) is called drug challenge, rechallenge. This is used throughout medical research to show causality of a drug or treatment.
Challenging Rhesus Monkeys with SARS-Cov-2 Virus
For example, a recent 2020 study by Dr. Chandrashekar, nine rhesus monkeys were challenged with SARS-Cov-2 virus by inoculating their nasal passages with the virus.(2) The challenged monkeys developed a mild upper respiratory disease with viral RNA detected in nasal samples. Additional blood testing showed the nine monkeys developed humoral and cellular immunity to the virus with both neutralizing antibodies and T-Cell immunity. On day 35 after the monkeys had recovered, they were re-challenged with the same dose of the SARS-Cov-2 virus, finding the monkeys were now immune and did not get sick. Thus, proving the title of the publication: “SARS-CoV-2 infection protects against rechallenge in rhesus macaques”. In other words, infection with the virus creates protective immunity which then prevents re-infection after re-exposure. This is the same idea of “herd immunity” in a population when enough people have protective immunity, the virus has no place to go and fizzles out. In this above example, the principle of challenge , re-challenge was used in a medical research study to show causality, infection with the virus causes protective immunity. Of course, rhesus monkeys are not humans so one might argue the findings may not hold true in humans. A study to verify these same finding in humans is easy enough and should be done.
Challenge Rechallenge for Hydroxychloroquine (HCQ)
What if we did a challenge/ rechallenge experiment on a population exposed to circulating Corona Virus (Sars-Cov2). What if we challenged the population with a drug, then dechallenged by stopping the drug, and then rechallenged by restarting the drug ? This is exactly what was unintentionally done in Switzerland where hydroxychloroquine (HCQ) was freely available. Use was temporarily suspended for three weeks, and then reinstated representing challege, dechallenge and rechallenge. See below chart.
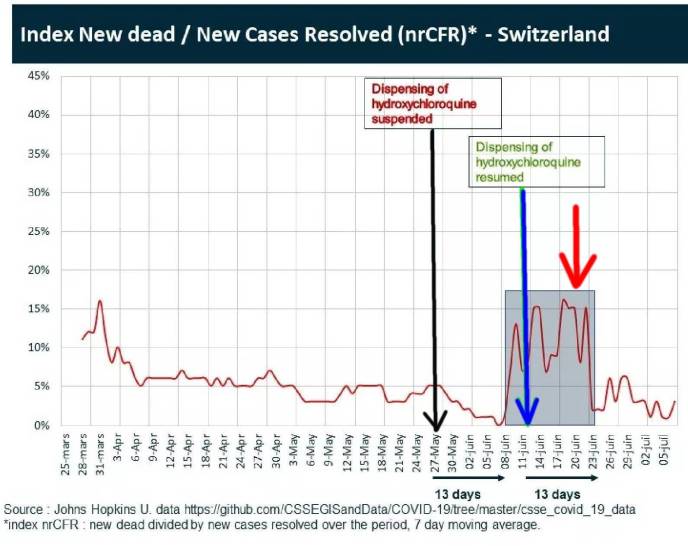
Horizontal red line is Swiss mortality data from COVID-19. Black Vertical Line: May 27 HCQ suspended. Blue Vertical Line HCQ reinstated. Red Vertical Line Increased Mortality from Covid-19 during window when HCQ use was suspended. This is highly significant !!!! Courtesy of Johns Hopkins University.
Hydroxychloroquine had been available and widely used in Switzerland until use was suspended on May 27, 2020 a few days after the negative May 22 Lancet study that prompted the WHO to suspend its drug trials. However, this Lancet study was found to be fraudulent, and was retracted on June 4. And, one week later on June 11, Switzerland reinstated use of Hydroxychloroquine. During this 20 day window, a dramatic increase in mortality from COVID-19 is observed (RED ARROW), showing challenge/rechallenge evidence indicating causality that removing the drug caused increased mortality from the virus.
Comparing CFR Case Fatality Rate to HCQ Use by Country
Folks if the above data from Switzerland doesn’t convince you, then look at this next chart, Covid-19 (CFR) Case Fatality Rate by HCQ use. The bar chart shows countries with no HCQ use (red), mixed use (orange) and free use (green). Left axis is CFR (case fatality rate).
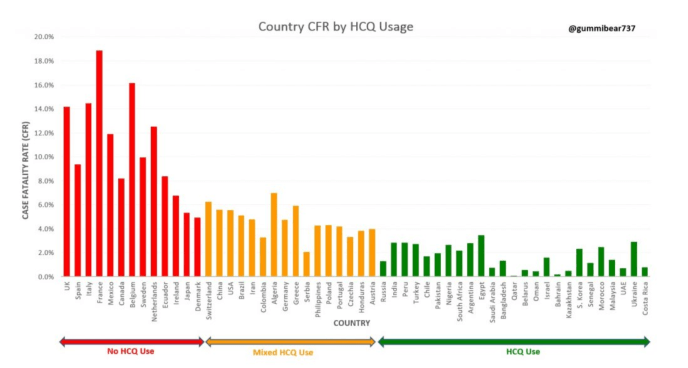
Covid-19 (CFR) Case Fatality Rate Correlates with HCQ Use
Red bar chart: NO HCQ use.
Orange Bar Chart: Mixed Use
Green Bar Chart /: Unrestricted Free HCQ Use – Much Lower Mortality Rate
This represents an observational study clearly demonstrating the efficacy of HCQ Hydroxychloroquine for reducing case fatality rate (CFR) for SARS-Cov-2 (COVD-19).
One Tenth the Mortality Rate in Countries Using HCQ
General Counsel Andrew Schlafly of the AAPS (American Association of Physicians and Surgeons) filed a court case against the FDA regarding restrictions on use of hydroxychloroquine. Mr. Schlafly says:
“The mortality rate from COVID-19 in countries that allow access to HCQ is only one-tenth the mortality rate in countries where there is interference with this medication, such as the United States….. In some areas of Central America, officials are even going door to door to distribute HCQ….These countries have been successful in limiting the mortality from COVID-19 to only a fraction of what it is in wealthier countries.” (12) End Quote Andrew Schlafly, General Counsel AAPS. (12)
Why Dr Fauci Insists on A Double Blind Placebo Controlled Study Before Endorsing HCQ for Covid19
Above White House News Conference from March 20, 2020
Remember the above exchange between President Trump and Dr Fauci during a white house Corona Virus Press Briefing (March 20, 2020)? President Trump said he had a “good feeling” about HCQ. However, Dr Fauci disagreed, claiming we need a randomized placebo controlled trial before recommending HCQ for Covid-19. This answer does indeed reveal that Dr Fauci represents the financial interests of the drug/vaccine industry, same as all other regulatory agencies, the CDC, FDA and NIH all captured by the drug industry many years ago. This is old news. In addition, Dr. Fauci knows that if President Trump ever “requests his resignation”, a lucrative job with the Gates Foundation, Merck, Pfizer or others is waiting with a paycheck at least ten fold greater than his current one, same as former FDA commissioner Dr. Scott Gottlieb who is now a board member for Pfizer, and biotech company Illumina. Same as Dr. Julie Gerberding, former director of the CDC (Centers for Disease Control and Prevention) , who is now president of Merck’s vaccine division.
Using a False Claim to Defeat the Competition
To make the claim that only a randomized placebo controlled drug trial can be accepted as “evidence” is not only a false claim, it is standard gimmick or ploy used by the pharmaceutical industry for decades. This claim maintains the only acceptable “evidence” is a randomized placebo controlled trial typically used for new drug FDA approval. Just by coincidence this excludes off-label repurposed drugs as well as natural substances that can not be patented.
Compounded Bioidentical Hormones Are Also on the Choppng Block
By the way, this includes bioidentical hormones since they can not be patented. The Pharmaceutical Industry has launched an insidious new campaign to ban compounded bioidentical hormones based on this same argument, namely the claim that only randomized placebo controlled trials represent “medical evidence”. The FDA which has been captured by the drug industry funded a report by the NASEM (National Academy of Science Engineering and Medicine) to come up with the predestined conclusion they wanted, that there is no evidence for efficacy or safety for bioidentical hormones, and therefore should be placed on the FDA “Difficult to Compound” List, a regulatory move which essentially bans compounded bioidentical hormones, eliminating a major economic competitor. It’s just business. Two consequences are 1) women will have no access to the hormone treatment they are currently using. 2) Compounding pharmacies across the nation will go out of business (13-14)
Dr Fauci Represents the Interests of the Drug Industry
As we discussed above, Dr. Fauci knows all this, including the fact that no drug company will ever fund such an expensive drug trial without the guarantee of profits through patent protection. If such a study is done by the drug industry or the government, it will be done with the express purpose of discrediting the drug. In the case of HCQ, this drug is especially odious to the drug industry since it competes with the potential billion dollar windfall in vaccine development and sales. The rush to discredit HCQ prompted studies published in the Lancet and NEJM that were so sloppy and fraudulent that both were retracted, an embarrassment unprecedented in the history of medicine. These fraudulent studies were funded for the express purpose of discrediting HCQ in order to eliminate the financial competition to the future Sars-Cov2 vaccines in the pipeline.(5-8)
A commonly cited failed HCQ study is a 2020 study in JAMA by Dr. Borba in Brazil obviously intentionally designed to discredit the HCQ drug by using lethal, highly toxic doses of HCQ in patients on ventilators. (9) Similarly, other unethical medical studies around the globe used lethal doses of the drug given to end stage ventilator patients.(15) HCQ is best used early in the disease course at lower dosage, safely used for decades for malaria and auto-immune disease. Efficacy is enhanced in combination with Zinc and Azithromycin (Z-Pack). Oral intake of Vitamin D3, Vitamin A, and Vitamin C to bowel tolerance enhance the immune system. Botanicals such as Elderberry, Chinese Skullcap, and Licorice have known antiviral activity.
Censorship of Physicians Having Good Outcomes Prescribing HCQ for Covid-19
I have personally seen good outcomes with the use of Hydroxychloroquine, Z-pack (azithromycin) and Zinc for upper respiratory infection attributed to Corona Virus, Sars Cov-2 (Covid -19). In addition, many physicians are coming forward stating they have had considerable success with this drug combination with good patient outcomes. Here is one such group of “Front Line” physicians speaking in front of the Supreme Court. Their video received 17 million views before it was deleted by You Tube, Google, Facebook and Twitter in a bizarre act of censorship. You can watch this video here:
Doctor’s Press Conference Drs. Simone Gold, Bob Hamilton, Stella Immanuel, Dan Erickson, James Todaro, Joe Ladapo on the steps of Supreme Court, Washington DC talking about HCQ, Z pack and ZINC for Covid 19. This video was censored/deleted by FB, U tube and Twitter.(10-11)
America’s Frontline Doctors SCOTUS Press Conference Transcript of above video press conference.
Governors in Five States Interfere with HCQ Prescribing by Physicians
Governor Steve Sisolak (D-NV)
Gov. Gretchen Whitmer (D-MI) (later reversed)
Gov. Cuomo (D-NY)
Gov. Mike DeWine (R-Ohio) (later reversed)
Gov. Doug Ducey (R-Arizona)
In an unprecedented move, Governors of 5 states including Gov. Cuomo of New York have issued executive orders banning or restricting the use of hydroxychloroquine for Covid-19. This is unprecedented in the history of medicine that a Government official would use their office to “Practice of Medicine without a License” and interfere with the doctor patient relationship. Needless to say, this interferes with the long standing off- label prescribing practices of physicians. How many lives have been sacrificed by the misguided executive orders of government agencies? These criminal acts should be held accountable by the Inspector General, the Attorney General and the Department of Justice.
Conclusion: The Challenge Rechallenge Data from Switzerland, and the comparative country data on CFR (Case fatality rate) with HCQ and without HCQ shows impressive efficacy of HCQ for reducing case fatality rates CFR. The use of HCQ should be liberalized and made OTC Over-The-Counter. Government officials and agencies should halt any interference in physician Off Label prescribing of Hydroxychloroquine, or be held accountable by the Attorney General.
Censorship Unprecedented in our History
Board certified physicians are standing up at great personal risk to inform the American People of the efficacy of hydroxychloroquine (HCQ). The fact these physicians are being censored and deleted by the Big Tech social media giants is simply astounding, and undoubtedly has caused unnecessary suffering and loss of life. The social media giants involved in this irresponsible censorship campaign should be held accountable and should pay a price for their negligence.
Jeffrey Dach, MD
7450 Griffin Road Suite 180/190
Davie, Fl 33314
telephone: 954 792-4663
Effective Early Treatment for Corona Virus (Covid-19)
Covid-19 Once in a Century Fiasco in the Making
Corona Virus How to Protect Ourselves
Links and References:
1) Radley, David C., Stan N. Finkelstein, and Randall S. Stafford. “Off-label prescribing among office-based physicians.” Archives of internal medicine 166.9 (2006): 1021-1026.
2) Chandrashekar, Abishek, et al. “SARS-CoV-2 infection protects against rechallenge in rhesus macaques.” Science (2020).
3) Elliott, Steve, and Angus M. Sinclair. “The effect of erythropoietin on normal and neoplastic cells.” Biologics: targets & therapy 6 (2012): 163.
4) Cao, Yihai. “Erythropoietin in cancer: a dilemma in risk therapy.” Trends in Endocrinology & Metabolism 24.4 (2013): 190-199.
5) (RETRACTED) Mehra, Mandeep R., et al. “Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis.” The Lancet (2020). May 22, 2020
6) (Retracted) Mehra, Mandeep R., et al. “Retraction: Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. DOI: 10.1056/NEJMoa2007621.” (2020).
7) Lancet, NEJM retract studies on hydroxychloroquine for COVID-19
Publish date: June 4, 2020 By Marcia Frellick
The Lancet announced today that it has retracted a highly cited study that suggested hydroxychloroquine may cause more harm than benefit in patients with COVID-19. Hours later, the New England Journal of Medicine announced that it had retracted a second article by some of the same authors, also on heart disease and COVID-19.
8) Iacobucci, Gareth. “Covid-19: Validity of key studies in doubt after leading journals issue expressions of concern.” (2020).
9) Borba, Mayla Gabriela Silva, et al. “Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial.” JAMA network open 3.4 (2020): e208857-e208857.
10) Doctors Break Down COVID Response and the Demonization of HCQ, DOCTORS TELL ALL July 1, 2020 Julie Carr The Ohio Star
In this interview, Dr. Simone Gold and Dr. Dan Wohlgelernter discuss the country’s failure to protect the elderly from the coronavirus and also sort out information around Hydroxychloroquine.
11) The Key to defeating Codi19 Harvey Risch MD
“I am usually accustomed to advocating for positions within the mainstream of medicine, so have been flummoxed to find that, in the midst of a crisis, I am fighting for a treatment that the data fully support but which, for reasons having nothing to do with a correct understanding of the science, has been pushed to the sidelines. As a result, tens of thousands of patients with Covid-19 are dying unnecessarily. Fortunately, the situation can be reversed easily and quickly. I am referring, of course, to the medication hydroxychloroquine. When this inexpensive oral medication is given very early in the course of illness, before the virus has had time to multiply beyond control, it has shown to be highly effective, especially when given in combination with the antibiotics azithromycin or doxycycline and the nutritional supplement zinc.”
—Harvey A Risch, MD, PhD, Professor of Epidemiology in the Department of Epidemiology and Public Health at the Yale School of Public Health and Yale School of Medicine
12) July 22, 2020 More Evidence Presented for Why Hydroxychloroquine Should be Made Available, in a New Court Filing by AAPS
“The mortality rate from COVID-19 in countries that allow access to HCQ is only one-tenth the mortality rate in countries where there is interference with this medication, such as the United States,” explains Andrew Schlafly. “In some areas of Central America, officials are even going door to door to distribute HCQ,” Andrew Schlafly adds. “These countries have been successful in limiting the mortality from COVID-19 to only a fraction of what it is in wealthier countries.”
Not using HCQ, Z-pack, and Zinc ??? That would really help to reduce the volume, work load and the mortality they are talking about…
13) FDA Turns Its Back on Women Action Alert Alliance for Natural Health July 23, 2020
As we recently reported, the FDA is gearing up to issue a ban on compounded bioidentical hormone replacement therapy. The newest development is a report from the National Academies of Sciences, Engineering, and Medicine (NASEM), which falsely concluded that these critical medicines used by millions of women are a “public health concern.” We must use every avenue available to fight the FDA and prevent a ban on estriol, progesterone, and other compounded bioidentical hormones.
14) What’s at Stake in FDA’s Hormone Attack Action Alert Alliance for Natural Health January 23, 2020
15) Dr. Meryl Nass Discovers Hydroxychloroquine Experiments Were Designed to Kill COVID Patients – How Many Were Murdered?
Aug 3 , 2020 Health Impact News
Published on August 2nd, 2020 by
The post Hydroxychloroquine Efficacy Against Corona Virus appeared first on Jeffrey Dach MD.









Leave a Comment
You must be logged in to post a comment.