At a recent medical meeting I attended, “the danger of TSH suppression” was mentioned. The listening audience of doctors was advised not to suppress TSH with thyroid medication. We were advised to make sure the TSH always stays within the lab reference range.
Thyroid Excess and Thyrotoxicosis
Of course, we would all agree that too much thyroid medication causing thyroid excess and thyrotoxicosis is to be avoided, as this clearly is the most significant adverse effect of taking thyroid hormone pills. Taking too many pills is not a good thing, as this will cause a clinical syndrome known as thyrotoxicosis characterized by tachycaria, palpitations, insomnia, anxiety, panic attacks etc. The arrythmia, atrial fibrillation is the dreaded complication to be avoided.
Take the Time to Go Over Thyroid Excess List of Symptoms
We actually spend 5 or 10 minutes with each patient in the office talking about Thyroid excess signs and symptoms to look for, and we give each patient a print out of this list to take home and post on their bulletin board to read every day. If the patient experiences any symptoms of thyroid excess they are instructed to stop the thyroid pills. Patient selection is also a factor here, as a patient with early dementia could not be expected to have the cognitive function to recognize thyroid excess and stop their pill. The patient must be able and willing to play a role in their health care. Otherwise, they are not a candidate for natural thyroid tretement, and instead are referred to your local friendly endocrinologist for levothyroxine dosage and TSH in range.
Stop the Thyroid Pills
Thyroid excess is easily avoided by simply stopping the thyroid medication should signs of rapid resting heart rate occur. Symptoms will resolve within hours after stopping the natural thyroid pill. For T4 only levothyroxine, which has a longer half life, it takes longer for symptoms to resolve.
Is a low TSH indicative of Thyroid Excess ?
Mainstream endocrinology makes the assumption that a suppressed TSH indicates thyroid excess, and by definition, thyrotoxicosis. This is true for Graves disease and Toxic Nodular Goiter. However, for the vast majority of hypothyroid patients taking natural dessicated thyroid (Nature-throid- RLC labs) a suppressed TSH merely indicates adequate treatment dosage with full clinical benefit, and does not correspond with the clinical signs and symptoms (or laboratory findings) of thyroid excess.
Monitoring the Other Labs and the Patient
Of course, we always monitor Free T3, Free T4, and Thyroid antibody levels, as well as monitor the patients clinical status. If the patient has a Free T3 and Free T4 in the normal range, and has no tachycardia at rest or any other symptoms of thyroid excess, then a suppressed TSH indicates adequate thyroid treatment, and by no stretch of the imagination could the patient possibly be in a state of thyroid excess or thyrotoxiciosis.
Adverse Health Effects of TSH Suppression
Another point mentioned at the medical meeting to persuade us clinician not to suppress the TSH with thyroid mediation was the idea that there are adverse health consequences when the TSH is suppressed. The major one being loss of bone density, osteoporoais which is a real finding in long standing Graves Disease patients.
However numerous studies have examined this idea in hypothyroid patients treated with thyroid pills and found this to be a medical myth. In our patient population we see bone density improving, especially in women receiving bioidentical hormone replacement and bone supplements such as Vitamin D3, K2, magnesium and calcium..
As to the idea that there must be some adverse heath effects of a suppressed TSH, this has also been examined in two groups of patients on long term TSH suppressive doses of thyroid medication, and this idea found to be false. In patients after thyroidectomy for papillary thyroid cancer who are commonly treated with TSH suppressive doses of thyroxine, there have been no adverse effects noted in many long term studies. Similarly in patients with thyroid nodules treated with TSH suppression, there have no ill effects of TSH suppression.
TSH Receptor Mutation Requires TSH Suppressive doses of Thyroid Hormone
Another patient population are those patients with mutations in the TSH receptor genes. Peripheral thyroid hormone resistance may also occur.
Conclusion: Long term TSH suppression in the thyroid nodule and thyroid cancer patient is a common practice of mainstream endocrinology with no adverse effects reported in many studies.
Articles with Related Interest:
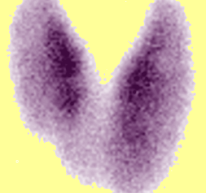
Above Left Image: Radionulcide Thyroid Scan showing diffuse goiter in patient with TSH receptor mutation and resistance to thyroid hormone courtesy of KIM.
Jeffrey Dach MD
7450 Griffin road Suite 190
Davie, Fl 33314
954-792-4663
Links and References
1) http://www.ncbi.nlm.nih.gov/pubmed/8954028?dopt=Abstract
J Clin Endocrinol Metab. 1996 Dec;81(12):4278-89.
Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. Uzzan B1, Campos J, Cucherat M, Nony P, Boissel JP, Perret GY.
1Hôpital Avicenne, Centre Hospitale Universitaire Paris Nord, France.
Osteoporosis is the main cause of spine and hip fractures. Morbidity, mortality, and costs arising from hip fractures have been well documented. Thyroid hormones (TH) are widely prescribed, mainly in the elderly. Some studies (but not all) found a deleterious effect of suppressive TH therapy on bone mass. These conflicting data raised a controversy as to the safety of current prescribing and follow-up habits, which, in turn, raised major health-care issues. To look for a detrimental effect on bone of TH therapy, we performed a meta-analysis (by pooling standardized differences, using a fixed effect model) of all published controlled cross-sectional studies (41, including about 1250 patients) concerning the impact of TH therapy on bone mineral density (BMD). Studies with women receiving estrogen therapy were excluded a priori, as were studies with a high percentage of patients with postoperative hypoparathyroidism, when no separate data were available. We decided to stratify the data according to anatomical site, menopausal status, and suppressive or replacement TH therapy, resulting in 25 meta-analysis on 138 homogeneous subsets of data. The main sources of heterogensity between studies that we could identify were replacement or suppressive TH therapy, menopausal status, site (lumbar spine, femoral neck, Ward’s triangle, greater trochanter, midshaft and distal radius, with various percentages of cortical bone), and history of hyperthyroidism, which has recently been found to impair bone mass in a large epidemiological survey. To improve homogeneity, we excluded a posteriori 102 patients from 3 studies, who had a past history of hyperthyroidism and separate BMD data, thus allowing assessment of the TH effect in almost all 25 subset meta-analyses. However, controls were usually not matched with cases for many factors influencing bone mass, such as body weight, age at menarche and at menopause, calcium dietary intake, smoking habits, alcohol intake, exercise, etc. For lumbar spine and hip (as for all other sites), suppressive TH therapy was associated with significant bone loss in postmenopausal women (but not in premenopausal women), whereas, conversely, replacement therapy was associated with bone loss in premenopausal women (spine and hip), but not in postmenopausal women. The detrimental effect of TH appeared more marked on cortical bone than on trabecular bone. Only a large long term prospective placebo-controlled trial of TH therapy (e.g. in benign nodules) evaluating BMD (and ideally fracture rate) would provide further insight into these issues.
2) http://www.ncbi.nlm.nih.gov/pubmed/15278189 J Formos Med Assoc. 2004 Jun;103(6):442-7. Bone mineral density in women receiving thyroxine suppressive therapy for differentiated thyroid carcinoma. Chen CH1, Chen JF, Yang BY, Liu RT, Tung SC, Chien WY, Lu YC, Kuo MC, Hsieh CJ, Wang PW.
Most patients with well-differentiated thyroid carcinoma have an excellent prognosis and are likely to live long enough to be subjected to osteoporosis. The purpose of this study was to investigate the consequences of treatment with a supraphysiological dose of levothyroxine (l-T4) on bone mineral density (BMD) in Taiwanese women with differentiated thyroid cancer.
METHODS:A total of 69 (44 premenopausal, 25 postmenopausal) Taiwanese women with differentiated thyroid cancer were included in this retrospective study. These patients were free of disease recurrence after initial near-total thyroidectomy and I-131 radioablation, and had undergone regular l-T4 suppressive therapy for more than 3 years (mean, 7.3 +/- 3.0 years; range, 3 to 15 years). The degree of thyroid-stimulating hormone (TSH) suppression was determined based on the mean TSH score for each patient which was determined by analysis of all available follow-up TSH data, where 1 = undetectable TSH (< 0.2 mIU/mL); 2 = subnormal TSH (0.2 to 0.39 mIU/mL); 3 = normal TSH (0.4 to 4.0 mIU/mL); and 4 = elevated TSH (> 4.0 mIU/mL). The patients were divided into a full TSH suppression group with a mean TSH score in the range 1.0 to 1.99, and a partial TSH suppression group with a mean TSH score in the range 2.0 to 2.99. BMD was measured by dual-energy X-ray absorptiometry at the lumbar spine, femoral neck, Ward’s triangle and total hip. Comparisons between subgroups of patients and controls were performed by unpaired t test. Correlation between BMD and other clinical variables was assessed by Pearson’s correlation analysis.
RESULTS:Postmenopausal patients (aged 57.7 +/- 6.9 years) had significantly higher serum calcium levels and decreased BMD at all sites of the spine and hip as compared with premenopausal patients (aged 38.6 +/- 6.7 years) with similar BMI and duration of TSH suppression. Comparison of BMD between postmenopausal patients and BMI- and age-matched controls revealed that the patient group had decreased BMD at all sites of measurement, although this difference was not significant. This phenomenon was not observed in the premenopausal patients. Furthermore, when BMD was compared between patients categorized as having full and partial suppression of TSH, only patients with full suppression in the postmenopausal group showed a tendency to lower BMD. There was a strong correlation of BMD with age, BMI and serum calcium level. However, no correlation was found between BMD and degree of TSH suppression or duration of l-T4 suppression therapy.
CONCLUSION: Women with differentiated thyroid cancer who had long-term (mean, 7.3 +/- 3.0 years) l-T4 therapy and suppressed TSH levels had no evidence of lower BMD. However, patients with full suppression in the postmenopausal group showed a tendency towards lower BMD. Therefore, careful monitoring of BMD in postmenopausal women during suppression therapy is mandatory.
3) http://www.ncbi.nlm.nih.gov/pubmed/20429634
Endocr Regul. 2010 Apr;44(2):57-63.
Thyrotropin versus thyroid hormone in regulating bone density and turnover in premenopausal women. Baqi L1, Payer J, Killinger Z, Hruzikova P, Cierny D, Susienkova K, Langer P.
This cross-sectional study aimed to evaluate the interrelations between endogenous TSH level on one side and the status of bone mineral density (BMD) and bone metabolic turnover (BMT) on the other in pooled four groups of premenopausal women either without or with a long-term L-thyroxine treatment.
METHODS:Serum levels of free thyroxine (FT4), thyrotropin (TSH), calcium (Ca), alkaline phosphatase (ALP), osteocalcin OC) and cross linked N-telopeptide of type 1 collagen (NTx) as well as urinary calcium (U-Ca/24h), bone mineral density of lumbar spine L 1-4 (BMD-L) and femoral hip (BMD-F) were estimated in a cohort of 151 premenopausal women (median 36 years) consisting of four groups: Group 1, 40 healthy untreated women, while three other groups consisted of patients previously treated for about 5 years; Group 2, 41 patients with genuine hypothyroidism treated by L-thyroxine (50-100 microg daily); Group 3, 40 patients with genuine hyperthyroidism treated by Carbimazol (10-15 mg daily); Group 4, 30 patients treated by suppressive doses of L-thyroxine (100-150 microg daily) after thyroidectomy for thyroid cancer (n=10) or because of progressively growing benign goitre (n=20).
RESULTS:When using multiple correlation analysis (Pearson’s r) in pooled 151 women, TSH showed significant positive correlation with BMD-L (p<0.01) and BMD-F (p<0.001) and, at the same time, significant negative correlation with serum level of BMT markers such as ALP (p<0.05), OC (p<0.05) and NTx (p<0.01), while the correlation of FT4 with BMD-L, BMD-F was significantly negative (p<0.001 for both) and that with all BMT markers was significantly positive (p<0.05 to <0.001). Thus, it appeared that higher TSH level was associated with increased bone mineral density and, at the same, with decreased bone metabolic turnover. These interrelations were further supported by the findings of significantly lower BMD-F (p<0.01), BMD-L (p<0.001) and significantly higher ALP, OC and NTX (all at p<0.001) in the group of 36 women with TSH level<0.3 mU/l as compared to the group of 115 women with TSH level range of 0.35-6.3 mU/l).
CONCLUSIONS:Irrespectively of thyroid diagnosis and/or previous long term thyroxine treatment in some groups, this cross sectional study showed that, after the pooled group of 151 women has been redistributed according to the actual TSH level, the bone mineral density and the level of bone turnover markers was significantly more favorable in 115 subjects with TSH level range of 0.35-6.3 mU/l than these in 36 women with TSH<0.3 mU/l.
4) http://www.ncbi.nlm.nih.gov/pubmed/3295553
N Engl J Med. 1987 Jul 9;317(2):70-5.
Suppressive therapy with levothyroxine for solitary thyroid nodules. A double-blind controlled clinical study. Gharib H, James EM, Charboneau JW, Naessens JM, Offord KP, Gorman CA.
Thyroid nodules are present in up to 50 percent of adults in the fifth decade of life. Patients are often treated with thyroxine in order to reduce the size of the nodule, but the efficacy of thyrotropin-suppressive therapy with thyroxine remains uncertain. In this study, 53 patients with a colloid solitary thyroid nodule confirmed by biopsy were randomly assigned in a double-blind manner to receive placebo (n = 25) or levothyroxine (n = 28) for six months. Before treatment, pertechnetate-99m thyroid scanning showed that 22 percent of the nodules were functional, 25 percent hypofunctional, and 53 percent nonfunctional. High-resolution (10-MHz) sonography was used to measure the size of the nodules before and after treatment. Suppression of thyrotropin release was confirmed in the levothyroxine-treated group by the administration of thyrotropin-releasing hormone; thyrotropin release was normal in the placebo group. Six months of therapy did not significantly decrease the diameter or volume of the nodules in the levothyroxine group as compared with the placebo group. We conclude that the efficacy of levothyroxine therapy in reducing the size of colloid thyroid nodules is not apparent within six months, despite effective suppression of thyrotropin.
5) http://www.ncbi.nlm.nih.gov/pubmed/12414852
J Clin Endocrinol Metab. 2002 Nov;87(11):4928-34.
Effects of thyroid-stimulating hormone suppression with levothyroxine in reducing the volume of solitary thyroid nodules and improving extranodular nonpalpable changes: a randomized, double-blind, placebo-controlled trial by the French Thyroid Research Group. Wémeau JL1, Caron P, Schvartz C, Schlienger JL, Orgiazzi J, Cousty C, Vlaeminck-Guillem V.
The efficacy of suppressing TSH secretion with levothyroxine (L-T(4)) in reducing solitary thyroid nodule growth is still controversial. In this prospective multicenter, randomized, double-blind, placebo-controlled trial, 123 patients with a single palpable benign nodule were included and randomly allocated to an 18-month treatment with L-T(4) or placebo. Individual dose was adjusted to allow a serum TSH level below 0.3 mIU/liter. Clinical and ultrasonographic nodule characteristics were assessed before treatment and 3, 6, 12, and 18 months thereafter. The largest mean nodule size assessed on palpation and largest volume, assessed by ultrasonography, decreased in the L-T(4) group and increased slightly in the placebo group [size, -3.5 +/- 7 mm vs. +0.5 +/- 6 mm (P = 0.006); volume, -0.36 +/- 1.71 ml vs. +0.62 +/- 3.67 ml (P = 0.01), respectively]. The proportion of clinically relevant volume reduction (> or =50%) rose significantly in the L-T(4) group [26.6% vs. 16.9% (P = 0.04)]. The proportion of patients with a reduced number of infraclinical additional nodules was significantly higher in the L-T(4) group [9.4% vs. 0 (P = 0.04)]. It is concluded from this study that suppressive L-T(4) therapy is effective in reducing solitary thyroid nodule volume and improving infraclinical extranodular changes.
6) http://www.ncbi.nlm.nih.gov/pubmed/1366242
Ir J Med Sci. 1992 Dec;161(12):684-6. TSH as an index of L-thyroxine replacement and suppression therapy. Igoe D1, Duffy MJ, McKenna TJ.
When hypothalamic-pituitary function is normal, serum TSH levels measured by ultrasensitive assay yield bioassays of endogenous thyroid action and thus provide an ideal index of thyroid secretion and its relationship to fluctuating endogenous thyroid levels. It is theoretically possible that patients receiving exogenous L-thyroxine for primary hypothyroidism should have suppressed TSH levels if physiological needs are constantly met. To examine this possibility free thyroxine, FT4 and TSH were measured in 90 clinically euthyroid patients receiving treatment with L-thyroxine for primary hypothyroidism. TSH levels were normal in 44, suppressed in 16 and elevated in 30 patients. FT4 levels were normal in 68, elevated in 13 and suppressed in 9 patients. Normal TSH levels were associated with normal FT4 levels in 79.5% of patients, elevated FT4 levels in 13.6% and low FT4 in 6.8%. Suppressed TSH levels were associated with elevated FT4 levels in 37.5% of patients and normal FT4 levels in 62.5%. When FT4 levels were normal, however, TSH levels were normal in only 51.5% and abnormal in 48.5%. We also examined the possibility that FT4 levels may remain within normal range when TSH is suppressed during L-thyroxine treatment for goitre or cancer. FT4 and TSH were measured in 45 patients on L-thyroxine as TSH suppression treatment. TSH was suppressed in 23 patients (51.1%), normal in 20 (44.4%) and elevated in 2 (4.5%). When TSH was suppressed, FT4 was elevated in 30.4% but normal in 69.6% of patients.(ABSTRACT TRUNCATED AT 250 WORDS)
7) http://press.endocrine.org/doi/full/10.1210/jcem.83.11.5215
Suppressive Therapy with Levothyroxine for Solitary Thyroid Nodules: A Double-blind Controlled Clinical Study and Cumulative Meta-analyses
Flávio Zelmanovitz 2 , Sandra Genro, and Jorge L. Gross
JCEM July 01, 2013
The data obtained in this study do not suggest any significant decrease in BMD after 1 yr of treatment with suppressive doses of T4. However, a meta-analysis of related studies, performed by Uzzan et al., demonstrated that suppressive therapy decreased the BMD in 409 postmenopausal patients after an average of 9.6 yr (11).
8) http://www.ncbi.nlm.nih.gov/pubmed/12803168
Ann Intern Med. 2001 Apr 3;134(7):561-8.
Risk for fracture in women with low serum levels of thyroid-stimulating hormone.
Bauer DC1, Ettinger B, Nevitt MC, Stone KL; Study of Osteoporotic Fractures Research Group.
Biochemical evidence of hyperthyroidism may be associated with low bone mass, particularly in older postmenopausal women, but no prospective studies of thyroid function and subsequent fracture risk have been done.
OBJECTIVE:To examine the association between low levels of thyroid-stimulating hormone (TSH) and fracture in older women.
DESIGN:Prospective cohort study with case-cohort sampling.
SETTING:Four clinical centers in the United States.
PATIENTS:686 women older than 65 years of age from a cohort of 9704 women recruited from population-based listings between 1986 and 1988.
MEASUREMENTS:Baseline assessment of calcaneal bone mass, spine radiography, and history of thyroid disease. Spine radiography was repeated after a mean follow-up of 3.7 years; nonspine fractures were centrally adjudicated. Thyroid-stimulating hormone was measured in sera obtained at baseline from 148 women with new hip fractures, 149 women with new vertebral fractures, and a subsample of 398 women randomly selected from the cohort.
RESULTS:After adjustment for age, history of previous hyperthyroidism, self-rated health, and use of estrogen and thyroid hormone, women with a low TSH level (0.1 mU/L) had a threefold increased risk for hip fracture (relative hazard, 3.6 [95% CI, 1.0 to 12.9]) and a fourfold increased risk for vertebral fracture (odds ratio, 4.5 [CI, 1.3 to 15.6]) compared with women who had normal TSH levels (0.5 to 5.5 mU/L). After adjustment for TSH level, a history of hyperthyroidism was associated with a twofold increase in hip fracture (relative hazard, 2.2 [CI, 1.0 to 4.4]), but use of thyroid hormone itself was not associated with increased risk for hip fracture (relative hazard, 0.5 [CI, 0.2 to 1.3]).
CONCLUSIONS:Women older than 65 years of age who have low serum TSH levels, indicating physiologic hyperthyroidism, are at increased risk for new hip and vertebral fractures. Use of thyroid hormone itself does not increase risk for fracture if TSH levels are normal.
9) http://www.ncbi.nlm.nih.gov/pubmed/1286519
Clin Endocrinol (Oxf). 1992 Dec;37(6):500-3.
Morbidity in patients on L-thyroxine: a comparison of those with a normal TSH to those with a suppressed TSH. Leese GP1, Jung RT, Guthrie C, Waugh N, Browning MC.
Patients on L-thyroxine with a ‘suppressed’ TSH (< 0.05 mU/l) were compared to those in whom TSH was detectable but not elevated (0.05-4.0 mU/l), with regard to morbidity data.
DESIGN:Biochemical data from Tayside Thyroid Register was matched to hospital admissions data obtained from Health Board Statistics.
PATIENTS:The patients were identified from those registered on the computerized Tayside Register.
MEASUREMENTS:Serum T4 and TSH assays, clinical assessment scores, and admission records with regard to ischaemic heart disease, overall fractures, fractured neck of femur and breast carcinoma.
RESULTS:Over one year, 1180 patients on thyroxine replacement had clinical and biochemical assessment; 59% had a suppressed TSH and 38% ‘normal’ TSH. Patients with a suppressed TSH exhibited higher median serum thyroxine levels (146 nmol/l, range 77-252 vs 119 nmol/l, 58-224; P < 0.001). Patients under the age of 65 years on L-thyroxine had an increased risk of ischaemic heart disease compared to the general population (female 2.7 vs 0.7%, P < 0.001; male 6.4 vs 1.7%, P < 0.01), but the risk was no different between those with suppressed and normal TSH. There was no increase in risk for overall fracture, fractured neck of femur or breast carcinoma in those on thyroxine with suppressed or normal TSH.
CONCLUSION:Patients under the age of 65 years on L-thyroxine had an increased risk of ischaemic heart disease. There was no excess of fractures in patients on L-thyroxine even if the TSH is suppressed.
10) http://www.ncbi.nlm.nih.gov/pubmed/0009284722
J Clin Endocrinol Metab. 1997 Sep;82(9):2931-6.
Low thyrotropin levels are not associated with bone loss in older women: a prospective study. Bauer DC1, Nevitt MC, Ettinger B, Stone K.
The relationship between excess thyroid hormone and bone loss is controversial. To determine whether low TSH levels, indicating excessive thyroid hormone, are associated with low bone mass or accelerated bone loss in older women, we performed a prospective cohort study of 458 women over age 65 yr participating in the multicenter Study of Osteoporotic Fractures. Three hundred and twenty-three women were randomly selected from the entire cohort of 9704; an additional 135 randomly selected thyroid hormone users were studied. Medical history, medication use, and calcaneal bone mineral density (BMD) were assessed at the baseline visit. Serum was collected and stored at -190 C. Hip and spine BMD were measured approximately 2 yr later, and follow-up calcaneal and hip BMD measurements were obtained after mean follow-up periods of 5.7 and 3.5 yr, respectively. TSH levels were determined in baseline serum samples using a third generation chemiluminescent assay. After adjustment for age, weight, previous hyperthyroidism, and use of estrogen, bone loss over 4-6 yr was similar in women with low, normal, or high TSH. For example, femoral neck bone loss was -0.3%/yr (95% confidence interval, -0.8%, 0.3%) among women with low TSH (< or = 0.1 mU/L) and -0.5%/yr (95% confidence interval, -0.7%, -0.3%) in those with normal TSH (0.1-5.5 mU/L). There were no statistically significant differences in baseline bone mass of the calcaneus, spine, or femoral neck or trochanteric hip subregions. Baseline total hip BMD was 6% lower (P = 0.01) in women with low TSH. Similar results were obtained in analyses confined to women not taking estrogens. We found no consistent evidence that low TSH, a sensitive biochemical marker of excess thyroid hormone, was associated with low BMD or accelerated bone loss in older ambulatory women.
conclusion, in this prospective study of thyroid function and skeletal health in older women, we found no consistent evidence that low TSH was associated with low bone mass or accelerated bone loss.
11) http://www.ncbi.nlm.nih.gov/pubmed/7848399
JAMA. 1994 Apr 27;271(16):1245-9.
Thyroid hormone use and bone mineral density in elderly women. Effects of estrogen. Schneider DL1, Barrett-Connor EL, Morton DJ.
To determine the effect of long-term use of thyroid hormone on bone mineral density (BMD) in elderly women and the potential mitigating effects of estrogen replacement therapy.
DESIGN:Cross-sectional, community-based study.
SETTING:Rancho Bernardo, Calif.
PARTICIPANTS:A total of 991 white women aged 50 to 98 years who participated in a study of osteoporosis.
MAIN OUTCOME MEASURES:Bone mineral density at the ultradistal radius and midshaft radius using single-photon absorptiometry and at the hip and lumbar spine using dual-energy x-ray absorptiometry.
RESULTS:A total of 196 women taking thyroid hormone for a mean duration of 20.4 years were compared with 795 women who were not using thyroid hormone. Women taking daily thyroxine-equivalent doses of 200 micrograms or more had significantly lower BMD levels at the midshaft radius and hip compared with those taking less than 200 micrograms. Daily doses of 1.6 micrograms/kg and greater were associated with lower bone mass at all four sites compared with nonuse, whereas doses less than 1.6 micrograms/kg were not associated with lower BMD levels. These associations were independent of age, body mass index, smoking status, and use of thiazides, corticosteroids, and estrogen. Women taking both estrogen and a thyroid hormone dose of 1.6 micrograms/kg or greater had significantly higher BMD levels at all four sites than women taking the same thyroid hormone dose alone. Women taking both thyroid hormone and estrogen had BMD levels comparable with those observed in women taking only estrogen.
CONCLUSIONS:Long-term thyroid hormone use at thyroxine-equivalent doses of 1.6 micrograms/kg or greater was associated with significant osteopenia at the ultradistal radius, midshaft radius, hip, and lumbar spine. Estrogen use appears to negate thyroid hormone-associated loss of bone density in postmenopausal women.
Women taking both thyroid hormone and estrogen had BMD levels comparable with those observed in women taking only estrogen.
12) http://www.ncbi.nlm.nih.gov/pubmed/9240728
J Bone Miner Res. 1997 Jan;12(1):72-7.
Skeletal integrity in men chronically treated with suppressive doses of L-thyroxine. Marcocci C1, Golia F, Vignali E, Pinchera A.
We measured bone mineral density (BMD) (lumbar spine, femoral neck, Ward’s triangle, and trochanter) in 34 men given suppressive doses of levothyroxine (L-T4) for a mean of 10.2 years. Indications for treatment were nontoxic goiter (n = 5) or thyroidectomy for differentiated thyroid cancer (n = 6) or nontoxic goiter (n = 3). Patients were followed at our institution and treated with the minimal amount of L-T4 able to suppress thyroid-stimulating hormone (TSH). At the time of evaluation, free T3 was normal in all cases, whereas free T4 was increased in 14 men (41.2%). The mean daily dose of L-T4 was 172 +/- 6 microg, and the cumulative dose of L-T4 was 673 +/- 71 mg. We found no significant difference between patients and age- and weight-matched controls in BMD (g/cm2) at any site of measurement (lumbar spine 1.144 +/- 0.12 vs. 1.168 +/- 0.15; femoral neck 0.979 +/- 0.13 vs. 1.001 +/- 0.13; Ward’s triangle 0.854 +/- 0.17 vs. 0.887 +/- 0.15; and trocanther 0.852 +/- 0.13 vs. 0.861 +/- 0.13). BMD was not correlated with the duration of therapy, cumulative or mean daily dose of L-T4, serum levels of free T4, free T3, osteocalcin, and bone alkaline phosphatase. Serum calcium and osteocalcin were slightly but significantly elevated in patients compared with controls, whereas there was no difference in intact parathyroid hormone, bone alkaline phosphatase, and sex hormone-binding globulin (marker of thyroid hormone action). Our data suggest that L-T4 suppressive therapy, if carefully carried out and monitored, using the smallest dose necessary to suppress TSH secretion, has no significant effects on bone metabolism and bone mass in men.
13) http://www.ncbi.nlm.nih.gov/m/pubmed/8157704/
Carefully monitored levothyroxine suppressive therapy is not associated with bone loss in premenopausal women. Marcocci C, et al. J Clin Endocrinol Metab. 1994.
We measured total body and regional (lumbar spine, femoral neck, Ward’s triangle, and trochanter) bone mineral density (BMD) in 47 premenopausal women chronically treated with suppressive doses of levothyroxine (L-T4). Treatment was administered to 7 patients with nontoxic goiter or, after thyroidectomy, to 38 patients with differentiated thyroid cancer and 2 with nontoxic goiter. Patients were followed at our institution and treated with the minimal amount of L-T4 necessary to suppress TSH. At the time of evaluation, free T3 was normal in all cases, whereas free T4 was increased in 17 (36.2%). The mean daily dose of L-T4 was 154.3 +/- 5 micrograms, and the mean duration of treatment was 10.1 yr. We found no significant difference between patients and age- and weight-matched controls in BMD at any site of measurement. BMD was not correlated with duration of therapy, cumulative or mean daily dose of L-T4, serum levels of free T4, free T3, and osteocalcin. There was no difference between patients and controls in serum total calcium, intact PTH, osteocalcin, or carboxy-terminal cross-linked telopeptide of type I collagen or in the concentrations of two markers of thyroid hormone action (sex hormone-binding globulin and amino-terminal propeptide of type III procollagen). Our data suggest that L-T4 suppressive therapy, if carefully carried out and monitored, using the smallest dose necessary to suppress TSH secretion has no significant effect on bone metabolism or bone mass.
13) http://www.ncbi.nlm.nih.gov/pubmed/7647577
Thyroid. 1995 Apr;5(2):81-7. Possible limited bone loss with suppressive thyroxine therapy is unlikely to have clinical relevance. Müller CG1, Bayley TA, Harrison JE, Tsang R.
To determine the effect of suppressive doses of thyroxine (T4) on bone mass, we studied 50 women on suppressive doses of T4 for 3-27 years (mean of 11 years). Twenty-five had nontoxic goiter and 25 had well-differentiated thyroid carcinoma. Fifty controls were matched for age, menopausal status, and body mass index. Bone mineral density (BMD) was measured in the lumbar spine (LS), femoral neck (FN), trunk (TK), and extremities (EXT) by dual-energy X-ray absorptiometry (DXA). In addition, the trunk area was measured by neutron activation analysis and recorded as a calcium bone index (CaBI). Twenty-one patients were restudied with DXA measurements at a mean of 1.5 +/- 0.5 (1 SD) years. The total population of 50 patients showed no difference in bone mass from controls. In patients with nontoxic goiter, there was no evidence of any loss in bone mass. Cancer patients showed insignificant reductions of 2-5% in BMD of LS, FN, and TK and a significant 5% reduction in BMD of EXT, compared to controls, and a 12% reduction in CaBI compared to goiter patients. Cancer patients had a slightly higher (p < 0.001) mean daily dose of T4 than goiter patients (0.23 vs 0.15 mg/day) but had a similar degree of TSH suppression. BMD and CaBI values did not correlate with free T4 index) with the daily T4 dose, accumulative dose, or with duration of T4 therapy. There were no significant changes in bone mass in either goiter or cancer patients restudied after a mean of 1.5 years.
14) http://www.ncbi.nlm.nih.gov/pubmed/7787427
Thyroid. 1995 Feb;5(1):13-7. Suppressive doses of thyroxine do not accelerate age-related bone loss in late postmenopausal women.
Fujiyama K1, Kiriyama T, Ito M, Kimura H, Ashizawa K, Tsuruta M, Nagayama Y, Villadolid MC, Yokoyama N, Nagataki S.
To examine whether suppressive doses of thyroxine have any adverse effects on bone, we evaluated various bone metabolic markers (lectin-precipitated alkaline phosphatase, osteocalcin, carboxyl-terminal region of type I collagen propeptide, tartrate-resistant alkaline phosphatase, and urinary excretion of hydroxyproline and pyridinium crosslinks), incidence of vertebral deformity, total body and regional (lumbar spine and radius) bone mineral densities (BMDs), and rates of bone loss in 24 late postmenopausal (more than 5 years after menopause) women who were treated with levothyroxine (L-T4) after total thyroidectomy for differentiated carcinoma. Depending on the clinical records, including serum TSH levels measured by immunoradiometric assay, these patients were divided into two groups. One group of patients was given suppressive doses of L-T4 (TSH < 0.1 mU/L, n = 12) and the other group was given nonsuppressive doses of L-T4 (TSH > 0.1 mU/L, n = 12). There was no difference in bone metabolic markers and incidence of vertebral deformity between the groups. In patients with TSH suppression, Z-scores of BMDs calculated from age-matched healthy women (n = 179, aged 55 to 80) were nearly in the zero range of values (0.077 at total body, 0.228 at lumbar spine, and -0.117 at trabecular region of lumbar spine). The rate of bone loss in TSH-suppressed patients (-0.849 +/- 0.605%/year) was not significantly different from that of nonsuppressed patients (-0.669 +/- 0.659). These prospective and cross-sectional data suggest that long-term levothyroxine therapy using suppressive doses has no significant adverse effects on bone.
15) http://www.ncbi.nlm.nih.gov/pubmed/8252740
Clin Endocrinol (Oxf). 1993 Nov;39(5):529-33.
Suppressed TSH levels secondary to thyroxine replacement therapy are not associated with osteoporosis. Grant DJ1, McMurdo ME, Mole PA, Paterson CR, Davies RR.
Recent studies have suggested that patients receiving thyroxine are at increased risk of osteoporosis. We set out to measure bone mineral densities in two groups of post-menopausal women receiving thyroxine replacement therapy (those with serum TSH levels persistently suppressed or non-suppressed) and to compare the results in both groups with those of the local control population.
DESIGN:Cross-sectional study.
PATIENTS:Seventy-eight post-menopausal women who had been treated with thyroxine for primary autoimmune or idiopathic hypothyroidism for a minimum of 5 years, 44 with TSH persistently suppressed and 34 non-suppressed. One hundred and two control subjects.
MEASUREMENTS:Forearm bone mineral density at proximal and distal sites as measured by single-photon absorptiometry.
RESULTS:Results were expressed as Z-scores, i.e. number of standard deviations from the mean of a 5-year age-band from the local control population. Mean Z-scores at proximal and distal sites for the non-suppressed patients were -0.03 and -0.07 and for the suppressed patients were -0.20 and -0.25, representing a decrease in bone mineral density of at most 5% in the suppressed patients. The differences between the three groups were not statistically significant.
CONCLUSION:In this patient population, the reduction in bone mineral density due to thyroxine is small. It is unlikely to be of clinical significance and should not on its own be an indication for reduction of thyroxine dose in patients who are clinically euthyroid.
16) http://erc.endocrinology-journals.org/content/12/4/973.long
Lack of deleterious effect on bone mineral density of long-term thyroxine suppressive therapy for differentiated thyroid carcinoma
J L Reverter, S Holgado1, N Alonso, I Salinas, M L Granada2 and A Sanmartí
Department of Endocrinology and Nutrition, Germans Trias i Pujol Hospital, Carretera de Canyet s/n. 08916, Badalona, Barcelona, Spain
1Department of Rheumatology, Germans Trias i Pujol Hospital, Badalona, Barcelona, Spain
2Hormone Laboratory, Germans Trias i Pujol Hospital, Badalona, Barcelona, Spain
The effect of subclinical hyperthyroidism on bone mineral density is controversial and could be significant in patients with differentiated thyroid carcinoma who receive suppressive doses of levothyroxine (LT4). To ascertain whether prolonged treatment with LT4 to suppress thyrotropin had a deleterious effect on bone mineral density and/or calcium metabolism in patients thyroidectomized for differentiated thyroid cancer we have performed a cross-sectional study in a group of 88 women (mean ± SD age: 51 ± 12 years) treated with LT4 after near-total thyroidectomy and in a control group of 88 healthy women (51 ± 11 years) matched for body mass index and menopausal status. We determined calcium metabolism parameters, bone turnover marker N-telopeptide and bone mass density by dual-energy X-ray absorptiometry. No differences were found between patients and controls in calcium metabolism parameters or N-telopeptide except for PTH, which was significantly increased in controls. No differences were found between groups in bone mineral density in femoral neck (0.971 ± 0.148 gr/cm2 vs 0.956 ± 0.130 gr/cm2 in patients and controls respectively, P = 0.5). In lumbar spine, bone mineral density values were lower in controls than in patients (1.058 ± 0.329 gr/cm2 vs 1.155 ± 0.224 gr/cm2 respectively, P<0.05). When premenopausal (n = 44) and postmenopausal (n = 44) patients were compared with their respective controls, bone mineral density was similar both in femoral neck and lumbar spine. The proportion of women with normal bone mass density, osteopenia and osteoporosis in patient and control groups was similar in pre- and postmenopausal women. In conclusion, long-term suppressive LT4 treatment does not appear to affect skeletal integrity in women with differentiated thyroid carcinoma.
17) http://www.ncbi.nlm.nih.gov/pubmed/21247815
Endocrinol Nutr. 2011 Feb;58(2):75-83. doi: 10.1016/j.endonu.2010.09.007. Epub 2011 Jan 17.
[Potential risks of the adverse effects of thyrotropin suppression in differentiated thyroid carcinoma].
[Article in Spanish]
Reverter JL1, Colomé E.
In patients with differentiated thyroid carcinoma, long-term inhibition of thyrotropin (TSH) secretion through levothyroxine administration is required when there is evidence of persistent or recurrent disease. In these cases, levothyroxine doses should be monitored to achieve the objectives of inhibiting TSH and avoiding clinical hyperthyroidism. The possibility that suppressive therapy may produce deleterious effects is still controversial, mainly in elderly patients. There are many studies on the potential harmful effects of suppressive therapy on various organs and systems with discrepant results. However, there is no scientific evidence that the clinical impact of these effects is significant.
18) http://www.ncbi.nlm.nih.gov/pubmed/20494634
Endocrinol Nutr. 2010 Oct;57(8):350-6. doi: 10.1016/j.endonu.2010.03.015. Epub 2010 May 24.
[Clinical endocrinologists’ perception of the deleterious effects of TSH suppressive therapy in patients with differentiated thyroid carcinoma].
[Article in Spanish]
Reverter JL1, Colomé E, Puig Domingo M, Julián T, Halperin I, Sanmartí A.
To explore the opinion of clinical endocrinologists as to the deleterious effects of thyrotropin (TSH) suppressive therapy in patients with differentiated thyroid carcinoma (DTC).
MATERIALS AND METHODS:
A self-administered survey was sent by e-mail to a group of endocrinologists with expertise in the treatment of patients with differentiated thyroid carcinoma. The questionnaire consisted of three questions related to: 1) the possible adverse effects of this therapy on different organ systems, 2) the clinical significance of these effects and 3) the usefulness of treatment guidelines for DTC.
RESULTS:A total of 91 endocrinologists responded with a wide divergence of opinions. No question had more than 80% of answers in a particular option. Of the possible side effects of suppressive therapy, a high degree of ignorance to three of them (increased left ventricular mass, reentrant tachycardia and diastolic dysfunction). Most respondents felt that the seven items, dementia and Alzheimer, decreased quality of life, decreased bone mineral density (BMD) in premenopausal women and men, thromboembolic disease, signs and symptoms of hyperthyroidism and increased risk of fractures were not affected by suppressive therapy, while most responded positively to two items (increased heart rate and decreased BMD in postmenopausal women). Eighty percent of the respondents felt that in any case these effects were not clinically significant and 33% considered that treatment guidelines should be reviewed.
CONCLUSIONS:Clinical endocrinologists seem to have a very heterogeneous opinion regarding the potential harmful effects of TSH-suppressive therapy for DTC.
19) http://www.townsendletter.com/Jan2011/ltrpub0111.html
Criminalizing Doctors Who Diagnose Hypothyroidism
The State of Oregon v. John E. Gambee, MD
John Gambee, MD is a physician practicing in Eugene, Oregon,
Letter from the Publisher
by Jonathan Collin, MD
20) http://thyroid.about.com/library/news/blderry2.htm
Dr. Derry Medical License Suspended
Copyright (c) 2015 Jeffrey Dach MD
The post TSH Suppression Benefits and Adverse Effects appeared first on Jeffrey Dach MD .









jeffrey_dach_md May 12, 2015 at 3:55 PM
Hashimotos patients are another group who may benefit from TSH supression…