Lithium Orotate NeuroProtection Part Two
by Jeffrey Dach MD
In part one, we discussed the differences between lithium carbonate, a prescription psychiatric drug, and a different version called lithium orotate. Unlike lithium carbonate, the lithium orotate is a safe nutritional supplement available without a prescription at the vitamin store. In Part Two, we review lithium as a neuro-protective agent. Link to Part One. Left image: Bottle Cap lithiated soda pop courtesy of Adam Clark Estes Factually Gizmodo.
Add Lithium to My Soda Pop, Please
In 1929, a new soda pop was introduced, lemon lime soda containing lithium citrate invented by Charles Grigg. This lithium containing drink was marketed as a cure for post alcohol Hang-Overs. Their marketing slogan was “Take the Ouch out of Grouch”. Later, when this lithiated soda pop was renamed 7-Up, it really took off and became a commercial success. Many other lithium containing beverages were popular during this era with names like “Wake-Up”, “Heads Up” and “B1″ (thiamine B1 was added). However, in 1948, 7-Up was reformulated without the added lithium and lithiated soda pop was removed from the market.(31-33). Left Images courtesy of 7-UP.
Another Medical Dogma Dies
 In the 1970’s, I learned in medical school the prevailing dogma that our brain cells start with a fixed number at birth which gradually declines as we age. Unlike other parts of the body which regenerate new cells, the brain is unable to replace lost or damaged brain cells. Or so we thought. Like many other discarded medical dogmas, this one has also fallen by the wayside. Just like other areas of the body, the brain also makes new brain cells. In the 1990’s, newer imaging techniques revealed neurogenesis in the hippocampus and olfactory bulb, specific areas of the brain where new cells are generated.(19-20) Left upper image MRI of brain for measuring grey matter volume courtesy of Wayne State University School of Medicine Dr Moore, October 7, 2000, The Lancet.(5)
In the 1970’s, I learned in medical school the prevailing dogma that our brain cells start with a fixed number at birth which gradually declines as we age. Unlike other parts of the body which regenerate new cells, the brain is unable to replace lost or damaged brain cells. Or so we thought. Like many other discarded medical dogmas, this one has also fallen by the wayside. Just like other areas of the body, the brain also makes new brain cells. In the 1990’s, newer imaging techniques revealed neurogenesis in the hippocampus and olfactory bulb, specific areas of the brain where new cells are generated.(19-20) Left upper image MRI of brain for measuring grey matter volume courtesy of Wayne State University School of Medicine Dr Moore, October 7, 2000, The Lancet.(5)
Lithium and Neurogenesis in the Hippocampus
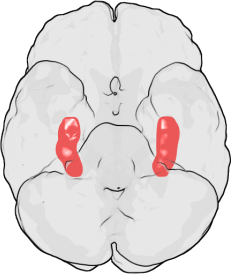 Research over the last decade has revealed that lithium’s effects as mood stabilization and regeneration of new brain cells (neurogenesis), are mainly due to the inhibition of a key enzyme called GSK-3β, glycogen synthase kinase-3β. (1) This GSK-3B, in turn, upregulates insulin-like growth factor-I (IGF-I), and brain-derived neurotrophic factor (BDNF) which stimulates neural stem cells to give rise to new hippocampal neurons throughout adulthood.(1) Thus, the molecular pathways of how lithium increases new brains cells has been worked out by basic science. Left Image: Location of Hippocamopus in human brain courtesy of wikimedia commons.
Research over the last decade has revealed that lithium’s effects as mood stabilization and regeneration of new brain cells (neurogenesis), are mainly due to the inhibition of a key enzyme called GSK-3β, glycogen synthase kinase-3β. (1) This GSK-3B, in turn, upregulates insulin-like growth factor-I (IGF-I), and brain-derived neurotrophic factor (BDNF) which stimulates neural stem cells to give rise to new hippocampal neurons throughout adulthood.(1) Thus, the molecular pathways of how lithium increases new brains cells has been worked out by basic science. Left Image: Location of Hippocamopus in human brain courtesy of wikimedia commons.
Lithium Increases Size of Hippocampus
One of the surprising findings in Bipolar Disorder recently made visible by new MRI brain imaging techniques is the loss of hippocampus neurons, and loss of brain volume characteristic of Bipolar disorder. This is also replicated in a mouse model of bipolar disorder in which GSK-3B is upregulated in genetically altered mice. These mice exhibit manic behavior and loss of brain volume which is reversed by lithium treatment.(7-8).
Neurotrophic Theory of Depression
Lithium prevents and reverses the loss of brain cells in Bipolar Disorder humans as demonstrated by MRI brain imaging studies. Brain volume increases after lithium treatment.(5-6)(9-10) Similar increases in brain volume have been seen with SSRi antidepressant drugs, suggesting a common mode of action with Lithium. This has launched the “neurotrophic theory of depression”.(21-23) This idea is new and may replace the older theory of neurotransmitter imbalances as a cause of depression. The Neurotrophic theory says that clinical depression is caused by reduced or absent brain neurogenesis, rather than chemical imbalances in the brain. The brain cannot make new cells. Lithium and SSRI antidepressants (24) increase these neurotrophic factors (by inhibiting GSK-3B) thus up-regulating neurogenesis in the hippocampus (26-29). Left Images:Seven-Up bottles lithiated lemon lime courtesy of Print MAg.
Lithium in Parkinson’s and Neurodegenerative Disorders and Traumatic Brain Injury,
Dr’s Lazzara and Kim reported in 2015 that lithium could potentially halt or reverse the neurodegeneration in Parkinson’s Disease(4) and in Alzheimer’s Dementia.(2-3) Lithium was also found protective in stroke and traumatic brain industry animal models models. Left image: Parkinson’s Disease Courtesy of PRemedHQ.
Lithium for Alzheimer’s
In a 2014 report, Dr Trujillo-Estrada laments that there is no effective treatment for Alzheimer’s dementia.(11) Dr Trujillo’s group studies the beneficial effects of lithium in a genetically modified mouse model of Alzheimer’s in which the mice exhibited ” prominent amyloid pathology along with a selective and significant neuronal loss in the hippocampus and entorhinal cortex.” The authors found less toxic plaque formation in the brains of lithium treated mice with improved memory function compared to placebo treated mice. The authors thought this was highly significant for Alzheimer’s Disease prevention, early treatment with lithium arrested neuron cell loss in the hippocampus and (olfactory – entorhinal cortex) in this mouse model.
Lithium for Traumatic Brain Injury
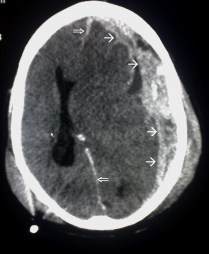 There are about 1.7 million people in the US with traumatic brain injury. Traumatic brain injury is considered a “signature wound” for returning military personnel fighting modern warfare. (13)
There are about 1.7 million people in the US with traumatic brain injury. Traumatic brain injury is considered a “signature wound” for returning military personnel fighting modern warfare. (13)
Left Image Traumatic Brain Injury CAT Scan showing left subdural hematoma (arrows) Courtesy of wikimedia.
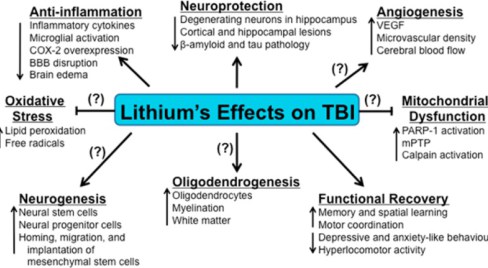
In 2014, Dr. Peter Leeds reviewed the neuroprotective effects of lithium in humans, and explored lithium neuroprotection in the mouse model of traumatic brain injury.(13) Dr Leeds summarizes his exciting finding here:
“Lithium demonstrated robust beneficial effects in experimental models of Truamatic Brain Injury (TBI). These include decreases in TBI-induced brain lesion, suppression of neuroinflammation, protection against blood-brain barrier disruption, normalization of behavioral deficits, and improvement of learning and memory, among others. ” (13)
 Lithium in Stroke and Brain Ischemia Models
Lithium in Stroke and Brain Ischemia Models
In an 2007 animal study of brain ischemia, Dr Yan reports lithium facilitated recovery of brain functions like spatial learning and memory in mice after cerebral ischemia caused by 15 minutes of carotid artery occlusion. (28) The authors state: “These results suggest that lithium up-regulates the generation and survival of new-born cells in the hippocampus ….and improves the behavioral disorder in rats after transient global cerebral ischemia.”(28)
Lithium – Why Isn’t Everyone Taking It ?
Conclusion: The body of evidence for the neuroprotective benefits of lithium is now overwhelming. Considering what we know about lithium, the only question I have is, “Why isn’t everyone taking it?” Again, I would suggest using the over the counter mineral form of lithium called lithium orotate. This is a nutritional supplement sold as capsules at most health food stores, previously available as a lemon soda pop called 7-UP.
Buy Lithium Orotateon Amazon.
This article is part two, for part one, click here.
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Fl 33314
954-792-4663
References
Neuroprotection of Lithium inhibition of GSK-3β
1) Lithium Neuropsychiatric Neuroplasticity Glycogen Synthase Kinase3_Wada_2009. Wada, Akihiko. “Lithium and Neuropsychiatric Therapeutics: Neuroplasticity via Glycogen Synthase Kinase-3. BETA.,. BETA.-Catenin, and Neurotrophin Cascades.” Journal of pharmacological sciences 110.1 (2009): 14-28.
Since 2004, it has been documented that among the multiple direct target molecules of lithium identified so far (e.g., inositol monophosphatase),
the beneficial effects of lithium, such as mood stabilization, behavioral amelioration, and neurogenesis, are due to the inhibition of glycogen synthase kinase-3β (GSK-3β) by lithium, which promotes β-catenin–dependent transcriptional events
new trends in the last five years have revealed that insulin-like growth factor-I (IGF-I), IGFII, and insulin enhance mood (9 – 12), memory (13, 14),
neurogenesis (13, 14), and angiogenesis (15); antidepressants up-regulate expression of IGF-I (16 – 18) and IGF-II (19), while IGF-I up-regulates brain-derived neurotrophic factor and its receptor TrkB
GSK-3, a serine/threonine protein kinase, controls multiple aspects of physiological events (e.g., cell membrane signal-to-gene transcription/protein translation, cytoskeletal organization, neuronal polarity, and cell survival /apoptosis)
Dysregulated hyperactivity of GSK-3β is associated with insulin resistance, diabetes mellitus, tumorigenesis, inflammation, and neuropsychiatric and
neurodegenerative diseases
inhibition of GSK-3β by various therapeutics (e.g.,lithium) allows β-catenin–induced de novo synthesis of IGF-I, IGF-II, brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor (VEGF).
Lithium: mood stabilization via GSK-3β inhibition and β-catenin activation
Therefore, therapeutic serum range (0.5 to 1.2 mM) of lithium inhibits GSK-3β in vivo.
Prickaerts et al. (47) documented that transgenic mice overexpressing GSK-3β mimicked hyperactivity as observed in the manic phase of bipolar disorder (e.g., increased locomotor activity), with reduction in brain weight;
Neurogenesis via GSK-3β inhibition and β-catenin activation.
Neural stem cells give rise to new hippocampal neurons throughout adulthood; conversely, defective neurogenesis may predispose an individual to mood disorders
In Caenorhabditis elegans, McColl et al. (52) documented that lithium elongated the life-span of the nematode; lithium down-regulated expression of histone
demethylase via mechanisms including GSK-3β inhibition by lithium.
Lithium treatment in Alzheimer’s patients: prevention of dementia
In a study of 1423 outpatients at a university psychiatric clinic, Terao et al. (63) reported that lithium therapy prevented the dementia in Alzheimer’s disease,
when their cognition and memory capacity were evaluated by Mini-Mental State Examination.
In mice, Li et al. (56) showed that intraperitoneal injection of d-fenfluramine
(to stimulate serotonin secretion and block its reuptake) rapidly increased Ser9-phosphorylation of GSK-3β by up to 500% at 1 h in the prefrontal cortex, hippocampus, and striatum.
Conclusions
There is to date compelling evidence indicating that the GSK-3β/β-catenin pathway is the convergent therapeutic target of lithium and various classical neuropsychiatric drugs, ameliorating behavior, mood, anxiety,
cognition, and neurogenesis. brain-derived neurotrophic factor and vascular endothelial growth factor),
IGF-I has proved to be a crucial factor in mood disorders. Antidepressants up-regulate IGF-I and IGF-II levels in the brain, and physical exercise and a healthy diet increases IGF-I synthesis in the brain and transport
of peripheral circulating IGF-I to the brain via the bloodbrain barrier. Importantly, IGF-I up-regulates the levels of brain-derived neurotrophic factor and its receptor TrkB, increasing synthesis of its downstream synaptic proteins.
2) Nciri, Riadh, et al. “Chronic neuroprotective effects of low concentration lithium on SH-SY5Y cells: possible involvement of stress proteins and gene expression.” Neural regeneration research 9.7 (2014): 735.
chronic exposure to lithium induces adaptive changes in metabolism of SH-SY5Y cells including a higher cell growth rate and a better resistance to oxidative stress
3) Chi-Tso, C. H. I. U., and De-Maw CHUANG. “Neuroprotective action of lithium in disorders of the central nervous system.” Zhong nan da xue xue bao. Yi xue ban= Journal of Central South University. Medical sciences 36.6 (2011): 461.
This article reviews recent findings regarding potential target involved in lithium’s neuroprotective effects and their implications fo the treatment of human disorders of the CNS.
Lithium’s main mechanisms of action appear to stem from its ability to inhibit glycogen synthase kinase-3 activity and also to induce signaling mediated by brain-derived neurotrophic factor.
Lithium in Parkinson’s and other NeuroDegenerative
4) full pdf free
Lazzara Kim Lithium in Parkinsons Neurodegenerative Dis Front Neurosci 2015
Lazzara, C. A., and Y. H. Kim. “Potential application of lithium in Parkinson’s and other neurodegenerative diseases. Front. Neurosci. 9: 403. doi: 10.3389/fnins. 2015.00403 Potential application of lithium in Parkinson’s and other neurodegenerative diseases Carol A. Lazzar a and Yong-Hwan Kim* Department of Biological Sciences, Delaware State University.” (2015).
Lithium inhibits apoptosis in brain and increases grey matter
Lithium given to humans with bipolar – MRI imaging
5) Moore, Gregory J., et al. “Lithium-induced increase in human brain grey matter.” The Lancet 356.9237 (2000): 1241-1242.
Rodent studies have shown that lithium exerts neurotrophic or neuroprotective effects. We used three-dimensional magnetic resonance imaging and brain segmentation to study pharmacologically-induced increases in grey matter volume with chronic lithium use in patients with bipolar mood disorder. Grey-matter volume increased after 4 weeks of treatment. The increases in grey matter probably occurred because of neurotrophic effects.
Human bipolar study-Lithium increases Hippocampus size and Verbal Memory
6) Psychopharmacology (Berl). 2007 Dec;195(3):357-67. Epub 2007 Aug 20.
Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Yucel K1, McKinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, MacQueen GM.
1Department of Psychiatry and Behavioural Neurosciences, McMaster University, 100 West 5th Street, Hamilton, ON, L8N 3K7, Canada,
OBJECTIVES:To our knowledge, no longitudinal volumetric study has been performed in patients with BD, which would allow for an examination of whether lithium therapy used to treat BD can exert a long-term effect on hippocampal volume.
MATERIALS AND METHODS:We examined the effects of lithium on hippocampal volumes and recollective memory performance over a period of 2 to 4 years in 12 patients with BD who had never received pharmacotherapy before lithium initiation.
RESULTS:We found bilateral increases in volume of the hippocampus over time. We also found some evidence of improvement in verbal memory performance over the 4-year measurement period as assessed by the California Verbal Learning Test.
CONCLUSIONS:Consistent with preclinical literature supporting the neuroprotective effects of lithium, long-term treatment is associated with preservation of recollective memory function and increased hippocampal size in vivo.
Lithium given to mice
7) Shimomura, Atsushi, Ryuji Nomura, and Takao Senda. “Lithium inhibits apoptosis of mouse neural progenitor cells.” Neuroreport 14.14 (2003): 1779-1782.
8) Riadh, Nciri, et al. “Neuroprotective and neurotrophic effects of long term lithium treatment in mouse brain.” Biometals 24.4 (2011): 747-757.
.
Since the worldwide approval of lithium therapy in 1970, lithium has been used for its anti-manic, antidepressant, and anti-suicidal effects. The last decade has witnessed the following discoveries about its neuroprotective and neurotrophic properties, yet the therapeutic mechanisms at the cellular level remain not-fully defined. We have undertaken the present study to determine if chronic lithium treatment, at therapeutically relevant concentrations, exerts neurotrophic/neuroprotective effects in the mouse brain in vivo. For this purpose, 10 months aged mice were fed for 3 months on food pellets contained 1 g (L1 group) or 2 g (L2 group) lithium carbonate/kg, resulting in serum concentrations of 0.4 and 0.8 mM, respectively. The evaluation of lipid peroxidation level and the activities of catalase, superoxide-dismutase and glutathione-peroxidase showed that chronic Li administration, at therapeutic doses doesn’t induce oxidative stress in brain tissue. No changes in the expression levels of molecular chaperones, namely, the HSP70, and HSP90 heat shock proteins and the GRP94 glucose-regulated protein were detected. Moreover, this treatment has caused
(1) an increase in the relative brain weight
(2) a delay in the age induced cerebral glucose impairment
(3) an enhancement of the neurogenesis in hippocampus and enthorinal cortex highlighted by silver impregnation.
Under these experimental conditions, no modifications were observed in expression levels of GSK3 and of its downstream target β-catenin proteins. These results suggested that chronic Li administration, at therapeutic doses, has a neuroprotective/neurotrophic properties and its therapeutic mechanism doesn’t implicate GSK3 inactivation.
9) Machado‐Vieira, Rodrigo, Husseini K. Manji, and Carlos A. Zarate Jr. “The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis.” Bipolar disorders 11.s2 (2009): 92-109.
The present article: (i) reviews what has been learned regarding lithium’s neurotrophic effects since Cade’s original studies with this compound; (ii) presents human data supporting the presence of cellular atrophy and death in BD as well as neurotrophic effects associated with lithium in human studies; (iii) describes key direct targets of lithium involved in these neurotrophic effects, including neurotrophins, glycogen synthase kinase 3 (GSK-3), and mitochondrial/endoplasmic reticulum key proteins; and (iv) discusses lithium’s neurotrophic effects in models of apoptosis and excitotoxicity as well as its potential neurotrophic effects in models of neurological disorders.
First, human studies showing evidence for neural cell atrophy and loss in BD are described, as well as lithium’s ability to reverse these pathological findings.
Second, the potential roles of intracellular cascade systems in BD are described; these have been shown to directly regulate cell survival/death pathways and are directly targeted by lithium.
Yucel et al. showed increased bilateral hippocampal volume after 2–4 years of lithium treatment in previously drug-naïve BD subjects (10).
Also, a recent study using high-resolution volumetric MRI showed a direct therapeutic relevance of lithium neurotrophic effects in BD. It was observed that only lithium-responders showed increases in gray matter in the prefrontal areas (11).
Third, the neurotrophic effects of lithium are described in preclinical models in vivo and in vitro and in clinical studies, demonstrating lithium’s clinical relevance not only in BD, but as a potential neurotrophic agent for use in several neurological disorders.
The most replicated finding from structural neuroimaging studies is an association between lithium treatment and increased gray matter volume in brain areas implicated in emotional processing and cognitive control, such as the anterior cingulate gyrus, amygdala, and hippocampus, which suggests that lithium has considerable neurotrophic effects (24, 25).
human postmortem and imaging studies in BD suggest that neurotrophic effects play a critical role in lithium’s therapeutic effects.
Glycogen synthase kinase 3 (GSK-3) polymorphism in the GSK-3 gene was associated with earlier onset of BD.
GSK-3 was shown to be downregulated by lithium in diverse studies, inducing direct neuronal protection against different injuries (40) and providing new insights into lithium’s neurotrophic effects [reviewed in (41)]. Indeed, GSK-3 is potently inhibited by chronic administration of lithium (42–44);
10) Prog Neuropsychopharmacol Biol Psychiatry. 2008 Dec 12;32(8):1761-71.
Lithium: bipolar disorder and neurodegenerative diseases Possible cellular mechanisms of the therapeutic effects of lithium. Marmol F1.
Bipolar illness is a major psychiatric disorder that affects 1-3% of the worldwide population. Epidemiological studies have demonstrated that this illness is substantially heritable. However, the genetic characteristics remain unknown and a clear personality has not been identified for these patients.
The clinical history of lithium began in mid-19th century when it was used to treat gout. In 1940, it was used as a substitute for sodium chloride in hypertensive patients. However, it was then banned, as it had major side effects.
Cade 1949
In 1949, Cade reported that lithium could be used as an effective treatment for bipolar disorder and subsequent studies confirmed this effect. Over the years, different authors have proposed many biochemical and biological effects of lithium in the brain. In this review, the main mechanisms of lithium action are summarised, including ion dysregulation; effects on neurotransmitter signalling; the interaction of lithium with the adenylyl cyclase system; inositol phosphate and protein kinase C signalling; and possible effects on arachidonic acid metabolism. However, none of the above mechanisms are definitive, and sometimes results have been contradictory.
Recent advances in cellular and molecular biology have reported that lithium may represent an effective therapeutic strategy for treating neurodegenerative disorders like Alzheimer’s disease, due to its effects on neuroprotective proteins like Bcl-2 and its actions on regulators of apoptosis and cellular resilience, such as GSK-3.
===================
Lithium Neuroprotective in Alzheimers Dementia
11) Trujillo-Estrada, Laura, et al. “Lithium, as a neuroprotective therapy for Alzheimer’s disease pathology, modifies abeta plaque toxicity.” (2014).
Lithium, as a neuroprotective therapy for Alzheimer’s disease pathology, modifies abeta plaque toxicity Author: Trujillo-Estrada, Laura; De Castro, Vanesa; Jimenez, S; Sanchez-Varo, Raquel; Sanchez-Mejias, Elisabet; Vizuete, Marisa; Vitorica, Javier; Gutierrez, Antonia Abstract: BACKGROUND: Despite the relatively large information about the Alzheimer’s disease (AD) pathology, no effective disease-modifying treatment has been yet developed. Lithium, a primary drug to treat bipolar disorder, has been suggested as a potential treatment against AD. In this work we have evaluated whether lithium treatment could ameliorate the neuropathology progression of the transgenic PS1M146L/APPSwe-London mice. Unlike most transgenic animal models, which do not exhibit the neurodegenerative spectrum of disease observed in the patient population, this AD model exhibits a prominent amyloid pathology along with a selective and significant neuronal loss in the hippocampus and entorhinal cortex. Therefore, this model is highly valuable for evaluating the effectiveness of potential neuroprotective therapies for AD. METHODS: For lithium treatment, PS1/APP mice (3 month old at the beginning of treatment) were fed, ad libitum, with diet supplemented with lithium carbonate (1.2g/kg, Harlan, Spain). The treatment lasts 6 months. After behavioural studies, mice were anesthetized and brains dissected out (hippocampus and cortex). Hemibrains were processed for immunohistochemistry, stereological and image analysis quantification, and the other hemibrains for RT-PCR and Western blot studies. RESULTS: Our data demonstrate that chronic oral administration of lithium, before the pathology onset, resulted in less toxic plaque formation that significantly ameliorated the degenerative processes and behavioural/memory deficits occurring during disease progression in our PS1/APP model. Specifically, and of great relevance for AD prevention, early lithium intervention was able to arrest neuronal loss in hippocampus and entorhinal cortex of highly vulnerable populations. Besides, lithium reduced the axonal dystrophic pathology, associated to amyloid plaques, by increasing the Abeta compaction. Moreover, a significant lower accumulation of phospho-tau, LC3-II and ubiquitinated proteins was detected. Our study highlights that the switch of plaque quality by lithium could be mediated by astrocyte activation and the release of heat shock proteins, which concentrated in the core of the plaques. –
Lithium protects hypoxia ischemia brain in mice
12) Li, Hongfu, et al. “Lithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cells.” Journal of Cerebral Blood Flow & Metabolism 31.10 (2011): 2106-2115.
In summary, lithium conferred impressive, morphological long-term protection against neonatal HI, at least partly by inhibiting inflammation and promoting NSPC proliferation and survival.
Lithium Protects Traumatic Brain Injury
13) Leeds, Peter R., et al. “A new avenue for lithium: intervention in traumatic brain injury.” ACS chemical neuroscience 5.6 (2014): 422-433.
lithium has been shown to reduce neuronal death, microglial activation, cyclooxygenase-2 induction, amyloid-β (Aβ), and hyperphosphorylated tau levels, to preserve blood-brain barrier integrity, to mitigate neurological deficits and psychiatric disturbance, and to improve learning and memory outcome. Given that lithium exerts multiple therapeutic effects across an array of CNS disorders, including promising results in preclinical models of TBI, additional clinical research is clearly warranted to determine its therapeutic attributes for combating TBI. Here, we review lithium’s exciting potential in ameliorating physiological as well as cognitive deficits induced by TBI.
Lithium for Alzheimers
14) Forlenza, Orestes Vicente, Vanessa de Jesus Rodrigues De-Paula, and B. S. O. Diniz. “Neuroprotective effects of lithium: implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders.” ACS chemical neuroscience 5.6 (2014): 443-450. Neuroprotective lithium Alzheimers neurodegenerative Forlenza 2014
Conclusions: Converging lines of evidence derived from preclinical and clinical models support the rationale for the study of the protective effects of lithium in neuropsychiatric conditions associated with chronic degeneration of the central nervous system.
15) Diniz, Breno Satler, Rodrigo Machado-Vieira, and Orestes Vicente Forlenza. “Lithium and neuroprotection: translational evidence and implications for the treatment of neuropsychiatric disorders.” Neuropsychiatric disease and treatment 9 (2013): 493.
In the last two decades, a growing body of evidence has shown that lithium has several neuroprotective effects. Several neurobiological mechanisms have been proposed to underlie these clinical effects. Evidence from preclinical studies suggests that neuroprotection induced by lithium is mainly related to its potent inhibition of the enzyme glycogen synthase kinase-3β (GSK-3β) and its downstream effects, ie, reduction of both tau protein phosphorylation and amyloid-β42 production. Additional neuroprotective effects include increased neurotrophic support, reduced proinflammatory status, and decreased oxidative stress. More recently, neuroimaging studies in humans have demonstrated that chronic use is associated with cortical thickening, higher volume of the hippocampus and amygdala, and neuronal viability in bipolar patients on lithium treatment. In line with this evidence, observational and case registry studies have shown that chronic lithium intake is associated with a reduced risk of Alzheimer’s disease in subjects with bipolar disorder. Evidence from recent clinical trials in patients with mild cognitive impairment suggests that chronic lithium treatment at subtherapeutic doses can reduce cerebral spinal fluid phosphorylated tau protein. Overall, convergent lines of evidence point to the potential of lithium as an agent with disease modifying properties in Alzheimer’s disease.
16) Gray, Jason D., and Bruce S. McEwen. “Lithium’s role in neural plasticity and its implications for mood disorders.” Acta Psychiatrica Scandinavica 128.5 (2013): 347-361.
Lithium Increases Hippocampal Volume in Bipolar Disorder
17) Suwalska, A., et al. “Neuroprotective effect of lithium on hippocampal volumes in bipolar disorder independent of long-term treatment response.” Neuroprotective effect lithium hippocampal volumes bipolar disorder Suwalska 2013
Among BD participants with substantial illness burden, the group with no or limited lifetime exposure to Li had smaller hippocampal volumes than the
Li-treated BD participants, who had hippocampal volumes comparable to controls. These results raise the possibility that the effects of Li on hippocampal volumes may generalize to patients with neuropsychiatric illnesses other than BD.
Lithium in Cancer PAtients
18) Khasraw, Mustafa, et al. “Using lithium as a neuroprotective agent in patients with cancer.” BMC medicine 10.1 (2012): 131.
Lithium exerts neuroprotective effects and is associated with less cognitive loss in various brain-injury models, including after cranial irradiation [30, 31]. In addition, neural stem/progenitor cells positively respond to lithium treatment under basal conditions [32, 33]. In addition to evidence from animal studies, neuroimaging research in humans supports the observation that lithium exerts neuroprotective effects. One study used three-dimensional magnetic resonance imaging and brain segmentation to evaluate increases in the effect of lithium on grey-matter volume in patients with bipolar mood disorder. The use of 4 weeks of lithium treatment was shown to increase brain grey-matter content [34] and hippocampal volume [35]. The authors concluded that increases in grey matter probably occurred as a result of neurotrophic effects.
Lithium was found to protect irradiated hippocampal neurons in mice from apoptosis, resulting in better performance in learning and memory function [31]. Lithium is known to reduce oxidative stress, specifically via the glutathione system [36]. In bipolar disorder, lithium has been shown to prevent the loss of cortical grey matter that occurs as part of the neuroprogressive cascade in the disorder [37].
Lithium-induced neural progenitor proliferation in vitro suggests that similar effects might occur in vivo, and this action could also be related to its clinical efficacy [39]. In animal studies, the effect of lithium treatment is partly mediated by inhibiting inflammation and by promoting proliferation and survival of neural stem and progenitor cells [44]. Lithium was shown to increase progenitor, rather than stem-cell, proliferation in both non-ischemic and ischemic rat brains [44].
Glycogen synthase kinase (GSK)-3 has been shown to be an essential mediator of neural progenitors during brain development.
Clinical data on the potential neuroprotective effect of lithium
There are limited prospective clinical data on the use of lithium as a neuroprotectant. Several small imaging studies have shown that patients with bipolar disorder treated with long-term lithium therapy have fewer structural changes on brain imaging compared with patients with bipolar disorder of at least 2 years in duration who received lithium for less than 3 months. Patients with bipolar disorder who were not treated with lithium were found to have smaller left hippocampal volumes than controls (corrected P<0.05). The study included 17 patients with bipolar disorder who had at least 2 years of lithium therapy, compared with 12 patients with bipolar disorder who had less than 3 months of lifetime lithium exposure. The group treated with lithium had hippocampal volumes similar to those of 11 healthy controls and of young, lithium-naïve patients [56]. In a similar study, measurement of left prefrontal N-acetyl aspartate (NAA) levels was performed using magnetic resonance spectroscopy at 1.5 T. The study included 27 participants treated with lithium, 16 participants not treated with lithium (<3 months exposure) and 21 healthy controls. The non-lithium group had lower prefrontal NAA levels than the lithium-treated group (P<0.01) or control group (P<0.05) [57].
——————————————————
Adult Neurogenesis
19) Ernst, Aurélie, and Jonas Frisén. “Adult neurogenesis in humans-common and unique traits in mammals.” PLoS Biol 13.1 (2015): e1002045.
20) Falk, Anna, and Jonas Frisén. “New neurons in old brains.” Annals of medicine 37.7 (2005): 480-486. New neurons in old brains Anna Falk Frisen 2005
Altman and colleagues suggested in the 1960s, contrary to the dogma posed by the founding fathers of neuroscience, that neurons were added in the olfactory bulb and the hippocampus of adult rodents (1).
It was not until the 1990s, with the introduction of novel techniques and the unequivocal demonstration of adult-born neurons by many laboratories that this
concept gained full acceptance. About fifteen years ago the thymidine analogue 5- bromo-3’-deoxyuridine (BrdU) was introduced BrdU is also incorporated in the DNA of the dividing cell and can be visualized with immunohistochemical
techniques. Imaging studies have demonstrated changes in the volume of distinct brain areas in response to, for example, training of a certain task (15) and in
major depression (16).
In a seminal study in 1998 Eriksson and colleagues for the first time demonstrated neurogenesis in the adult human hippocampus by BrdU labeling (17). Therefore, the time of birth of a population of cells can be established retrospectively by the analysis of 14C in genomic DNA (7).
Stem cells in the adult brain The neurons generated in adulthood derive from
stem or progenitor cells. Neural stem cells are immature cells that have the potential to generate the main cell types of the central nervous system:
neurons, astrocytes and oligodendrocytes (23,24).
The neurons that continuously are added to the hippocampus derive from local resident stem cells present in the subgranular zone of the dentate gyrus
(24,34–36). The neurons that are added to the olfactory bulb derive from stem cells residing in the lateral wall of the lateral ventricles, from where they
migrate along the rostral migratory stream to the olfactory bulb (37–39).
Learning, enriched environment and physical activity stimulate the generation of adult born neurons in the hippocampus (48–50). Upon stress and ageing
the level of glucocorticoids is raised, and high levels of adrenal steroids decrease the number of proliferating cells in the dentate gyrus (51–54).
neurogenesis may be important for certain aspects of memory formation (59). Moreover, it was suggested that depression leads to reduced neurogenesis in the hippocampus, and that the behavioral effects of many antidepressants may
be mediated by the stimulation of neurogenesis in the hippocampus (16).
Our view of the adult brain has changed with the realization of the presence of endogenous stem cells and continuous neurogenesis in certain regions.
———————
Neurogenesis Hypothesis in Depression and Anxiety Disorders
21) http://www.ncbi.nlm.nih.gov/pubmed/21945290/
Neuropharmacology. 2012 Jan;62(1):21-34. doi: 10.1016/j.neuropharm.2011.09.003. Epub 2011 Sep 19. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building?
Petrik D1, Lagace DC, Eisch AJ.
Hypotheses are scaffoldings erected in front of a building and then dismantled when the building is finished. They are indispensable for the workman; but you mustn’t mistake the scaffolding for the building. Johann Wolfgang von Goethe. The neurogenesis hypothesis of affective disorders – in its simplest form – postulates that the generation of neurons in the postnatal hippocampal dentate gyrus is involved in the etiology and treatment efficacy of major depressive disorder (MDD). The hypothesis was established in the 1990s but was built on a broad foundation of earlier research on the hippocampus, serotonin and MDD. It has gone through several growth phases fueled by discoveries both correlative and causative in nature. Recently, the hypothesis has also been broadened to also include potential relevance for anxiety disorders, like post-traumatic stress disorder (PTSD). As any hypothesis should be, it has been tested and challenged, sometimes vigorously. Here we review the current standing of the neurogenesis hypothesis of affective and anxiety disorders, noting in particular how a central postulate – that decreased neurogenesis results in depression or anxiety – has, in general, been rejected. We also review the controversies on whether treatments for these disorders, like antidepressants, rely on intact neurogenesis for their efficacy, and the existence of neurogenesis-dependent and -independent effects of antidepressants. In addition, we review the implications that the hypothesis has for the response to stress, PTSD, and the neurobiology of resilience, and highlight our own work showing that adult-generated neurons are functionally important for the behavioral response to social stress. We conclude by emphasizing how advancements in transgenic mouse technology, rodent behavioral analyses, and our understanding of the neurogenesis process will allow us to refine our conclusions and perform ever more specific experiments. Such scrutiny is critical, since if we “mistake the scaffolding for the building” we could overlook opportunities for translational impact in the clinic. This article is part of a special Issue entitled ‘Anxiety and Depression’.
22) Eisch, Amelia J., and David Petrik. “Depression and hippocampal neurogenesis: a road to remission?.” Science (New York, NY) 338.6103 (2012): 72.
“the neurogenic hypothesis of depression”.
23) http://www.ncbi.nlm.nih.gov/pubmed/12946878/
Biol Psychiatry. 2003 Sep 1;54(5):499-503.
Depressed new neurons–adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Kempermann G1, Kronenberg G.
1Max Delbrück Center for Molecular Medicine Berlin-Buch, and Department of Experimental Neurology, Charité University Hospital, Humboldt University, Berlin, Germany.
In a novel theory, a failure of adult hippocampal neurogenesis has been proposed to provide the biological and cellular basis of major depression. The as yet unresolved function of the new hippocampal neurons will have to be in the center of any attempt to prove this hypothesis. Only knowledge of normal functional relevance of new neurons will allow an assessment of their potential role in disturbed hippocampal function in depression; however, major depression is not primarily a hippocampal disorder. We therefore propose that consideration of the neurogenesis hypothesis of depression be the most prominent aspect of a more general cellular plasticity hypothesis.
24) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3438317/
Boldrini, Maura, et al. “Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression.” Biological psychiatry 72.7 (2012): 562-571.
Antidepressants increase human hippocampal NPCs and angiogenesis selectively in the anterior and mid DG. These results raise the possibility of a causal relationship between angiogenesis and neurogenesis, as seen in other proliferating tissues, and support their possible role in the mechanism of action of antidepressants.
=======================================
25) http://ijnp.oxfordjournals.org/content/17/12/1923
Boldrini, Maura, et al. “Benzodiazepines and the potential trophic effect of antidepressants on dentate gyrus cells in mood disorders.” International Journal of Neuropsychopharmacology 17.12 (2014): 1923-1933.
Lithium
26) http://www.ncbi.nlm.nih.gov/pubmed/10987856
J Neurochem. 2000 Oct;75(4):1729-34.
Enhancement of hippocampal neurogenesis by lithium.
Chen G1, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK.
Increasing evidence suggests that mood disorders are associated with a reduction in regional CNS volume and neuronal and glial cell atrophy or loss. Lithium, a mainstay in the treatment of mood disorders, has recently been demonstrated to robustly increase the levels of the cytoprotective B-cell lymphoma protein-2 (bcl-2) in areas of rodent brain and in cultured cells. In view of bcl-2’s antiapoptotic and neurotrophic effects, the present study was undertaken to determine if lithium affects neurogenesis in the adult rodent hippocampus. Mice were chronically treated with lithium, and 5-bromo-2-deoxyuridine (BrdU) labeling of dividing cells was conducted over 12 days. Immunohistochemical analysis was undertaken 1 day after the last injection, and three-dimensional stereological cell counting revealed that lithium produced a significant 25% increase in the BrdU-labeled cells in the dentate gyrus. Double-labeling immunofluorescence studies were undertaken to co-localize BrdU-positive cells with neuron-specific nuclear protein and showed that approximately 65% of the cells were double-labeled. These results add to the growing body of evidence suggesting that mood stabilizers and antidepressants exert neurotrophic effects and may therefore be of use in the long-term treatment of other neuropsychiatric disorders.
27) http://www.ncbi.nlm.nih.gov/pubmed/15056276
J Neurochem. 2004 Apr;89(2):324-36.
Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo.
Kim JS1, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H.
Lithium has been demonstrated to increase neurogenesis in the dentate gyrus of rodent hippocampus. The present study was undertaken to investigate the effects of lithium on the proliferation and differentiation of rat neural progenitor cells in hippocampus both in vitro and in vivo. Lithium chloride (1-3 mM) produced a significant increase in the number of bromodeoxyuridine (BrdU)-positive cells in high-density cultures, but did not increase clonal size in low-density cultures. Lithium chloride at 1 mM (within the therapeutic range) also increased the number of cells double-labeled with BrdU antibody and TuJ1 (a class III beta-tubulin antibody) in high-density cultures and the number of TuJ1-positive cells in a clone of low-density cultures, whereas it decreased the number of glial fibrillary acidic protein-positive cells in both cultures. These results suggest that lithium selectively increased differentiation of neuronal progenitors. These actions of lithium appeared to enhance a neuronal subtype, calbindin(D28k)-positive cells, and involved a phosphorylated extracellular signal-regulated kinase and phosphorylated cyclic AMP response element-binding protein-dependent pathway both in vitro and in vivo. These findings suggest that lithium in therapeutic amounts may elicit its beneficial effects via facilitation of neural progenitor differentiation toward a calbindin(D28k)-positive neuronal cell type.
28) Yan, Xue-Bo, et al. “Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia.” Neuropharmacology 4.53 (2007): 487-495.
Recent studies have demonstrated that lithium has a neuroprotective effect against brain ischemia. Whether this effect is mediated by hippocampal neurogenesis remains unknown. The ERK (extracellular signal-regulated kinase) pathway plays an essential role in regulating neurogenesis. The present study was undertaken to investigate whether lithium regulates hippocampal neurogenesis by the ERK pathway and improves spatial learning and memory deficits in rats after ischemia. Rats were daily injected with lithium (1 mmol/kg) and 2 weeks later subjected to 15-min ischemia induced by four-vessel occlusion method. 5-bromo-2′-deoxyuridine (Brdu; 50mg/kg) was administrated twice daily at postischemic day 6, or for 3 days from postischemic day 6 to 8. We found that lithium increased the ERK1/2 activation after ischemia by western blotting analysis. There was a significant increase in Brdu-positive cells in the hippocampal dentate gyrus after lithium treatment, compared with ischemia group at postischemic days 7 and 21; furthermore, the survival rate of Brdu-positive cells was elevated by lithium. Inhibition of the ERK1/2 activation by U0126 diminished these effects of lithium. The percentages of Brdu-positive cells that expressed a neuronal marker or an astrocytic marker were not significantly influenced by lithium. Moreover, lithium improved the impaired spatial learning and memory ability in Morris water maze, and U0126 attenuated the behavioral improvement by lithium. These results suggest that lithium up-regulates the generation and survival of new-born cells in the hippocampus by the ERK pathway and improves the behavioral disorder in rats after transient global cerebral ischemia.
29) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2889681/
Quiroz, Jorge A., et al. “Novel Insights into Lithium’s Mechanism of Action: Neurotrophic and Neuroprotective Effects.” Neuropsychobiology 62.1 (2010): 50.
30) full free pdf Stress serotonin hippocampal neurogenesis in relation to depression antidepressant effects Mahar 2014
Mahar,Ian, et al. “Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects.” Neuroscience & Biobehavioral Reviews 38 (2014): 173-192.
Lithium Soft Drinks
31) El-Mallakh, Rif S., and Rona Jeannie Roberts. “Lithiated lemon-lime sodas.” The American journal of psychiatry 164.11 (2007): 1662. Lithiated lemon-lime sodas American J Psychiatry El Mallak Roberts 2007
32)
The Original 7-Up Was A Mind-Altering Substance. The Huffington Post By Alexander C. Kaufman 09/17/2014
33) 7 Up Lithium, and Depression William B Jensen
7 Up Lithium, and Depression William B Jensen pdf
link to this page: http://wp.me/p3gFbV-3iD
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydachmd.com
http://www.drdach.com
http://www.naturalmedicine101.com
http://www.bioidenticalhormones101.com
http://www.truemedmd.com
Disclaimer click here: http://www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2015 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
Serving Areas of: Hollywood, Aventura, Miami, Fort Lauderdale, Pembroke Pines, Miramar, Davie, Coral Springs, Cooper City, Sunshine Ranches, Hallandale, Surfside, Miami Beach, Sunny Isles, Normandy Isles, Coral Gables, Hialeah, Golden Beach ,Kendall,sunrise, coral springs, parkland,pompano, boca raton, palm beach, weston, dania beach, tamarac, oakland park, boynton beach, delray,lake worth,wellington,plantation.
The post Lithium Orotate NeuroProtection Part Two appeared first on Jeffrey Dach MD .









Leave a Comment
You must be logged in to post a comment.