 Cancer Immunotherapy with GcMAF – Restoring the Immune System
Cancer Immunotherapy with GcMAF – Restoring the Immune System
by Jeffrey Dach MD
This article is part two of a series
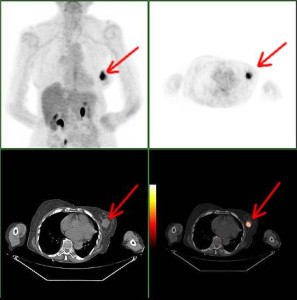
Left Image Breast Cancer (red arrow) on PET and CAT scans Courtesy of Wikimedia Commons
In part one of this series, we discussed a mouse with innate immunity to cancer by virtue of its immune cells which kill the cancer cells as if they were any other microbial invader. In this article, part two, we will expand on this idea by exploring the work of Dr. Yamamoto who discovered the Macrophage Activating Factor (GcMAF) in 1990 at the Socrates Institute in Philadelphia. (4) Since then, Dr. Yamamoto has published three human clinical trials showing remarkable results for breast(5), colo-rectal (10) and prostate cancer(11).
What is MAF – Macrophage Activating Factor?
MAF is a protein which activates our macrophages, the microscopic white cells that kill invading microbes and cancer cells. MAF is made from a precursor protein called the Gc protein.
 Cancer is Clever- It Inactivates Our Immune System
Cancer is Clever- It Inactivates Our Immune System
In a way, cancer cells are clever little devils because they disable our immune system, in order to enhance their own survival. Dr. Yamamoto discovered that cancer cells do this by secreting an enzyme called Nagalase which prevents the precursor protein Gc from being converted to MAF. This Nagalase enzyme activity can actually be measured in cancer patients, and greater tumor burden corresponds with higher Nagalase enzyme activity (as one would expect). Elimination of the tumor results in reduction of Nagalase activity to lower, more normal values.. (5)
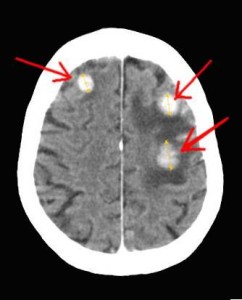
Left Image: CAT SCan Brain Showing enhancing metastatic lesions from breast cancer primary. Courtesy of wikimedia commons.
Dr. Yamamoto devised a technique for restoring Gc protein activity which creates the most potent macrophage activating factor ever discovered, having no adverse effects. He called it GcMAF. Macrophages treated in vitro with GcMAF (100 pg/ml) are highly effective at killing breast cancer cells.
GcMAF for Metastatic Breast Cancer-Human Trial
Dr. Yamamoto then studied his GcMAF in human metastatic breast cancer patients with weekly injections of 100 ng of GcMAF .(5) Dr. Yamamoto found that over time, as treatment with GcMAF progresses, the MAF precursor activity of patient Gc protein increased, and the serum Nagalase decreased.(5). After 5 months of weekly GcMAF injections , the cancer patients elevated Nagalase activity had returned to normal levels, same as healthy controls. Over the next four years, these sixteen treated metastatic Breast Cancer patients remained cancer free with no recurrence.(5) In 2008, Dr Yamamoto published his landmark study on human breast cancer.(5)
 GcMAF for Metastatic Colorectal Cancer – Human Trial
GcMAF for Metastatic Colorectal Cancer – Human Trial
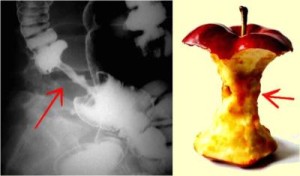
Left Image Red Arrow points to Colon Cancer with Apple Core Lesion on Barium Enema Xray courtesy of Radiopedia.org.
In 2008, Yamamoto published his study on 8 patients with metastatic colorectal cancer . They all had significant metastatic disease after primary resection.(10) Nagalase activity fell to normal levels with GcMAF injections, and remained low with no cancer recurrence over 7 years of observation. This was supported by serial CAT scans that remained negative.
 GcMAF for Metastatic Prostate Cancer – Human Trial
GcMAF for Metastatic Prostate Cancer – Human Trial
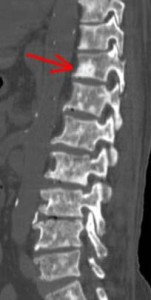
Left image: Prostate Cancer Bone Mets (Red Arrow)courtesy of Radiopedia.org
Dr. Yamamoto studied GcMAF in 16 patients with metastatic prostate cancer with excellent results, Nagalase activity declined to normal, and there was no evidence of tumor recurrence over 7 years of observation. (10)
“Sixteen nonanemic prostate cancer patients received weekly administration of 100 ng of GcMAF. As the MAF precursor activity increased, their serum Nagalase activity decreased. Because serum Nagalase activity is proportional to tumor burden, the entire time course analysis for GcMAF therapy was monitored by measuring the serum Nagalase activity. After 14 to 25 weekly administrations of GcMAF (100 ng/week), all 16 patients had very low serum Nagalase levels equivalent to those of healthy control values, indicating that these patients are tumor-free. No recurrence occurred for 7 years.”quote from abstract of 2008 paper(10)
Left Image: Dr Toshio Inui, MD.established Saisei Mirai Clinic in Kobe In 2010.
Patient 1. A 71-year-old man was diagnosed with thymic carcinoma with lung metastasis. The patient received 24 weeks of the integrative immunotherapy. No progression of the cancer was found 12 months after completion of the therapy.
Patient 2. A 74-year-old man was diagnosed with prostate cancer with multiple bone metastases. He received 12 weeks of the integrative immunotherapy combined with hyperthermia therapy. Bone scintigram results nine months after initiation of the therapy were normal and metastatic tumors had disappeared.
Patient 3. A 72-year-old woman was diagnosed with metastatic liver cancer after sigmoidectomy and bilateral oophorectomy. She received 24 weeks of the integrative immunotherapy combined with 55 Gy of radiation. There was no evidence of local recurrence or metastatic disease on Positron Emission Tomography (PET) and Computed Tomography (CT) scans 12 months after initiation of the therapy.
1) http://www.saisei-mirai.or.jp/
GcMAF (Gc Protein derived Macrophage Activating Factor) For the treatment for cancer, HIV and immune system diseases.
<<<<<<<<<< image >>>>>>>>>
Graduated from Kyoto Prefectural University of Medicine in 1978.
At the age of 33, after gaining experience working in the internal medicine department at a general hospital as a physician, he established Inui Clinic for the treatment of internal diseases.
He soon came to develop a reputation for careful and heartwarming medical examinations which made him popular with patients.
After the loss of his father due to cancer, he came to realize the limitations of conventional therapies for cancer such as surgery, chemothearapy anticancer drugs and radiation therapy, and he started his new practice specializing in immunotherapy for cancer. He also changed his clinic name to Inui Immunotherapy Cancer Clinic.
by Uto, Yoshihiro, Hitoshi Hori, Kentaro Kubo, Masamitsu Ichihashi, Norihiro Sakamoto, Martin Mette, Toshio Inui, and Trade Center. Nature 485 (2012): S67-S70.
http://www.ncbi.nlm.nih.gov/
Anticancer Res. 2013 Jul;33(7):2917-9.
Clinical Experience of Integrative Cancer Immunotherapy with GcMAF.
Inui T, Kuchiike D, Kubo K, Mette M, Uto Y, Hori H, Sakamoto N.
Division of Food and Drug Evaluation Science, Department of Community Medicine and Social Healthcare Science, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-cho, Chuo-ku, Kobe 650-0017,
PATIENTS AND METHODS:The standard protocol of our integrative cancer immunotherapy is as follows: i) 0.5 ml GcMAF-containing human serum is administered intramuscularly or subcutaneously once or twice per week for the duration of cancer therapy until all cancer cells are eradicated; ii) hyper T/natural killer (NK) cell therapy is given once per week for six weeks; iii) high-dose vitamin C is administered intravenously twice per week; iv) alpha lipoic acid (600 mg) is administered orally daily; v) vitamin D3 (5,000-10,000 IU) is administered orally daily.
RESULTS:By March 2013, Saisei Mirai have treated over 345 patients with GcMAF. Among them we here present the cases of three patients for whom our integrative immunotherapy was remarkably effective.
CONCLUSION:The results of our integrative immunotherapy seem hopeful. We also plan to conduct a comparative clinical study.>
It is our immune system that prevents and destroys disease
Int J Cancer. 2008 Jan 15;122(2):461-7.
Immunotherapy of metastatic breast cancer patients with vitamin D-binding protein-derived macrophage activating factor (GcMAF).by Yamamoto N, Suyama H, Yamamoto N, Ushijima N. Division of Cancer Immunology and Molecular Biology, Socrates Institute for Therapeutic Immunology, Philadelphia, PA 19126-3305, USA.
Anticancer Res. 2012 Jan;32(1):45-52.
Effects of vitamin D-binding protein-derived macrophage-activating factor on human breast cancer cells. Pacini S, Punzi T, Morucci G, Gulisano M, Ruggiero M.
Department of Anatomy, Histology and Forensic Medicine, Viale Morgagni 85, University of Firenze, Italy.
MATERIALS AND METHODS:The effects of DBP-MAF on proliferation, morphology, vimentin expression and angiogenesis were studied by cell proliferation assay, phase-contrast microscopy, immunohistochemistry and western blotting, and chorioallantoic membrane (CAM) assay.
RESULTS:DBP-MAF inhibited human breast cancer cell proliferation and cancer cell-stimulated angiogenesis. MCF-7 cells treated with DBP-MAF predominantly grew in monolayer and appeared to be well adherent to each other and to the well surface. Exposure to DBP-MAF significantly reduced vimentin expression, indicating a reversal of the epithelial/mesenchymal transition, a hallmark of human breast cancer progression.
CONCLUSION:These results are consistent with the hypothesis that the known anticancer efficacy of DBP-MAF can be ascribed to different biological properties of the molecule that include inhibition of tumour-induced angiogenesis and direct inhibition of cancer cell proliferation, migration and metastatic potential.
PLoS One. 2010 Oct 18;5(10):e13428. doi: 10.1371/journal.pone.0013428.
Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells.by Gregory KJ, Zhao B, Bielenberg DR, Dridi S, Wu J, Jiang W, Huang B, Pirie-Shepherd S, Fannon M.Department of Ophthalmology and Visual Sciences, University of Kentucky, Lexington, Kentucky
METHODS AND FINDINGS:In this study we show for the first time that DBP-maf exhibits a direct and potent effect on prostate tumor cells in the absence of macrophages. DBP-maf demonstrated inhibitory activity in proliferation studies of both LNCaP and PC3 prostate cancer cell lines as well as metastatic clones of these cells. Flow cytometry studies with annexin V and propidium iodide showed that this inhibitory activity is not due to apoptosis or cell death. DBP-maf also had the ability to inhibit migration of prostate cancer cells in vitro. Finally, DBP-maf was shown to cause a reduction in urokinase plasminogen activator receptor (uPAR) expression in prostate tumor cells. There is evidence that activation of this receptor correlates with tumor metastasis.CONCLUSIONS:These studies show strong inhibitory activity of DBP-maf on prostate tumor cells independent of its macrophage activation.
J Surg Res. 2012 Jan;172(1):116-22.
Nonaka K, Onizuka S, Ishibashi H, Uto Y, Hori H, Nakayama T, Matsuura N, Kanematsu T, Fujioka H.Clinical Research Center, National Hospital Organization Nagasaki Medical Center, Department of Hepatology, Nagasaki University Graduate School of Biomedical Sciences, Omura, Japan.
Bill Sardi Article
Transl Oncol. 2008 Jul;1(2):65-72.
Immunotherapy for Prostate Cancer with Gc Protein-Derived Macrophage-Activating Factor, GcMAF.by Yamamoto N, Suyama H, Yamamoto N. Source Division of Cancer Immunology and Molecular Biology, Socrates Institute for Therapeutic Immunology, Philadelphia, PA
Abstract Serum Gc protein (known as vitamin D(3)-binding protein) is the precursor for the principal macrophage-activating factor (MAF). The MAF precursor activity of serum Gc protein of prostate cancer patients was lost or reduced because Gc protein was deglycosylated by serum alpha-N-acetylgalactosaminidase (Nagalase) secreted from cancerous cells. Therefore, macrophages of prostate cancer patients having deglycosylated Gc protein cannot be activated, leading to immunosuppression. Stepwise treatment of purified Gc protein with immobilized beta-galactosidase and sialidase generated the most potent MAF (termed GcMAF) ever discovered, which produces no adverse effect in humans. Macrophages activated by GcMAF develop a considerable variation of receptors that recognize the abnormality in malignant cell surface and are highly tumoricidal. Sixteen nonanemic prostate cancer patients received weekly administration of 100 ng of GcMAF. As the MAF precursor activity increased, their serum Nagalase activity decreased. Because serum Nagalase activity is proportional to tumor burden, the entire time course analysis for GcMAF therapy was monitored by measuring the serum Nagalase activity. After 14 to 25 weekly administrations of GcMAF (100 ng/week), all 16 patients had very low serum Nagalase levels equivalent to those of healthy control values, indicating that these patients are tumor-free. No recurrence occurred for 7 years.Prostatic cancer diagnosis and prognosis have been aided by the availability of PSA measurement . When patients received radical prostatectomy, a sudden drop of high PSA levels to very low values was observed (Table 1). Thus, PSA is predominantly produced from primary tumor lesions in prostate compared with the metastasized lesions. Although serum Nagalase decreased during GcMAF therapy of patients with tumor-bearing prostate, PSA remained unchanged (Table 3). Therefore, PSA values cannot be used for prognostic assays during GcMAF therapy.2008 Yamamoto Human Trial GCMAF in Metastatic Colorectal Cancer
11) Yamamoto, Immunotherapy of metastatic colorectal Can Imm Imm 2008
http://www.ncbi.nlm.nih.gov/pubmed/18058096
Cancer Immunol Immunother. 2008 Jul;57(7):1007-16.
Immunotherapy of metastatic colorectal cancer with vitamin D-binding protein-derived macrophage-activating factor, GcMAF. by Yamamoto N, Suyama H, Nakazato H, Yamamoto N, Koga Y. Division of Cancer Immunology and Molecular Immunology, Socrates Institute for Therapeutic Immunology, 1040, 66th Ave, Philadelphia,
Serum vitamin D binding protein (Gc protein) is the precursor for the principal macrophage-activating factor (MAF). The MAF precursor activity of serum Gc protein of colorectal cancer patients was lost or reduced because Gc protein is deglycosylated by serum alpha-N-acetylgalactosaminidase (Nagalase) secreted from cancerous cells. Deglycosylated Gc protein cannot be converted to MAF, leading to immunosuppression. Stepwise treatment of purified Gc protein with immobilized beta-galactosidase and sialidase generated the most potent macrophage-activating factor (GcMAF) ever discovered, but it produces no side effect in humans. Macrophages treated with GcMAF (100 microg/ml) develop an enormous variation of receptors and are highly tumoricidal to a variety of cancers indiscriminately. Administration of 100 nanogram (ng)/ human maximally activates systemic macrophages that can kill cancerous cells. Since the half-life of the activated macrophages is approximately 6 days, 100 ng GcMAF was administered weekly to eight nonanemic colorectal cancer patients who had previously received tumor-resection but still carried significant amounts of metastatic tumor cells.

Jeffrey Dach MD
http://www.jeffreydach.com/
http://www.drdach.com/
http://www.naturalmedicine101.com/
http://www.truemedmd.com/
Disclaimer click here: http://www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media,
I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article: http://wp.me/p3gFbV-pr
Copyright (c) 2009-2013 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.









Leave a Comment
You must be logged in to post a comment.