 Heart Disease Vitamin C, Ascorbate, Lysine and Linus Pauling
Heart Disease Vitamin C, Ascorbate, Lysine and Linus Pauling
by Jeffrey Dach MD
The Steam Roller is Not Joking
I once had a conversation with a cardiologist friend of mine in which I casually mentioned the Linus Pauling Theory of heart disease, and the subsequent idea that a non-toxic nutritional supplement program with vitamin C and a few amino acids could prevent and reverse heart disease. The response from my cardiologist friend was hearty laughter that anyone would even suggest such a nonsensical idea, and surely you must be joking?
left image: courtesy wikimedia, roadroller
My cardiologist friend and the rest of the mainstream medical system has no clue about the steamroller (above image) coming to health care aiming right at the huge profits from diagnosis and treatment of heart disease, a multi billion dollar industry in the US.
The Cardiac Cath Lab and Cardiac Bypass Surgery Program will be flattened, the first casualties of the internet medical information revolution providing information about a safe and cheap supplement program to reverse heart disease called the Linus Pauling Protocol. Time has come for the old dinosaurs to go. In the near future, thanks to Linus Pauling, heart disease will become a curiosity of the past, like the disappearance of gastric ulcers after the invention of antacids and antibiotics.
The Linus Pauling Protocol – Vitamin C
 Vitamin C Deficiency Has Major Impact on Collagen Production
Vitamin C Deficiency Has Major Impact on Collagen Production
Vitamin C is required to make a protein called collagen which is the major component of connective tissues. The lack of Vitamin C is a deficiency disease called Scurvy.
Why is Collagen Important ?
Collagen is the most abundant protein in the body. It is the structural protein used to make connective tissues, bones, teeth, hair, and arteries. Strong collagen is important for a strong body.
Above Left image: “Unraveling Collagen“, Stainless Steel, height: 11 feet Julian Voss-Andreae, Orange Memorial Park Sculpture Garden, City of South San Francisco, CA. 5/10/2006. Courtesy of Wikimedia Commons
Vitamin C Deficiency Results in Absent Lysine Crosslinking on Collagen
Vitamin C is required for strong collagen. How does this work? Vitamin C is required for lysyl hydroxylase, an enzyme responsible for attaching the lysine residues together on adjacent collagen strands. (see diagram below) Vitamin C deficiency results in weakened collagen strands caused by disrupted lysine crosslinking. The resulting weakened collagen results in a widespread problems in the connective tissues, bones, teeth, skin, hair, arteries, etc.
Steps in Collagen Synthesis (see diagram below Courtesy of Wikimedia)
Fibril Cross Linking with Lysine and Lysyl Oxydase (see diagram below):
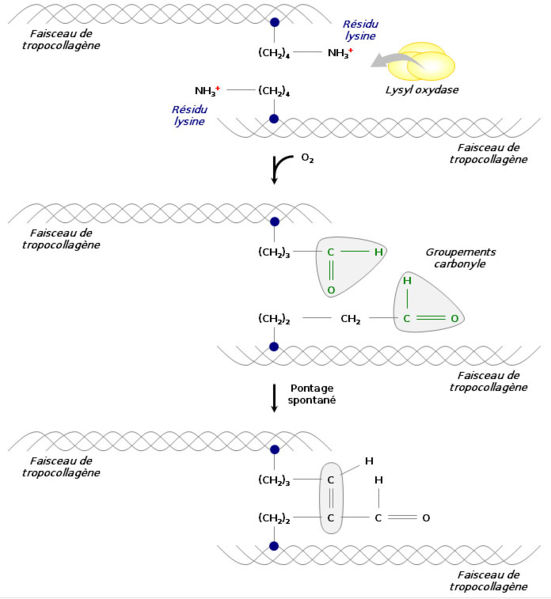 Above image courtesy of wikimedia commons lysyl oxidase.
Above image courtesy of wikimedia commons lysyl oxidase.Full Blown Scurvy – Collagen Falls Apart
In the full blown Vitamin C deficiency disease called Scurvy, the structural elements of the body literally fall apart. Collagen is broken down and not replaced. The joints wear out, the small arteries begin to crack and degenerate, the skin shows easy bruising and bleeding as small vessels rupture throughout the body, and the teeth may loosen and fall out.
Linus Pauling: Heart Disease is a Chronic Scurvy Condition
 Linus Pauling was unquestionably the greatest scientist of the twentieth century. All of modern biochemistry and molecular biology chemistry is based on Linus Pauling’s work, especially his discovery and elucidation of the chemical bond. Pauling is the only scientist to be awarded two unshared Nobel prizes.
Linus Pauling was unquestionably the greatest scientist of the twentieth century. All of modern biochemistry and molecular biology chemistry is based on Linus Pauling’s work, especially his discovery and elucidation of the chemical bond. Pauling is the only scientist to be awarded two unshared Nobel prizes.
Pauling’s later years were devoted to heart disease, and in 1989 he published “A Unified Theory of Human Cardiovascular Disease,” in which he states that atherosclerotic plaques in heart disease are actually part of a repair process, to repair the arterial damage caused by chronic vitamin C deficiency.
Left Image: Linus Pauling Courtesy of Wikimedia
In essence, Pauling said that heart disease is a manifestation of chronic scurvy, and atherosclerotic plaque is a mechanism evolved to repair or patch blood vessels and arteries damaged by chronic vitamin C deficiency. Linus Pauling also said that atherosclerotic plaque formation can be prevented or reversed with vitamin C, lysine and proline. These are nutritional supplements available at any health food store for a few dollars.
Atherosclerotic Plaques Contain LipoProtein (a)
Plaque deposits found in human aortas are made up of a form of cholesterol called lipoprotein (a) also called Lp(a).
Atherosclerotic Plaques Are Found at Maximal Mechanical Stress
Atherosclerotic plaques are not found randomly distributed throughout the arterial tree, rather distribution is restricted to sites of high mechanical stress such as bifurcations, and areas of motion such as the surface of the heart (coronary arteries). In the early 1950’s, a Canadian, G. C. Willis, MD, made these same observations, and they have been confirmed by 60 years of coronary and peripheral arteriography at major medical centers.
Exposed Lysine Crosslinks from Damaged Collagen is Site of LipoProtein (a) attachment and Plaque Formation
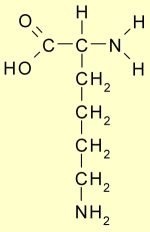 Imagine stepping on your garden hose a thousand times a day. You will soon notice cracks in the wall of the garden hose. This is the same process that happens in the artery. As these cracks open up, the collagen strands in the wall of the artery are teased apart. The triple helix collagen strands are normally bound together with lysine crosslinks which are now teased apart and exposed to the circulating blood stream.
Imagine stepping on your garden hose a thousand times a day. You will soon notice cracks in the wall of the garden hose. This is the same process that happens in the artery. As these cracks open up, the collagen strands in the wall of the artery are teased apart. The triple helix collagen strands are normally bound together with lysine crosslinks which are now teased apart and exposed to the circulating blood stream.
left Image: Lysine courtesy of WIkimedia
The Lysine residues look like little flags waving from the damaged collagen strand. The exposed Lysine strands are available for binding to circulating Lipoprotein (a), a special form of cholesterol that has lysine receptors, and is known to increase heart disease risk. This attachment of lipoprotein(a) to the free lysine residues of damaged collagen initiates the atherosclerotic process. Over time, this process builds larger plaque deposits which eventually narrow the inner diameter of the artery causing a blockage, or leads to plaque rupture and thrombosis, a catastrophic event which may cause heart attack or sudden death.
Animal experiments in genetically modified mice which have “knocked out” the lysine binding sites on lipoprotein (a) show a fivefold reduction in atherosclerotic plaque formation.
Can You Make Vitamin C ? No You Can’t
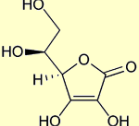 We humans cannot make vitamin C in our liver as all other animals do. We humans had a genetic mutation in our ancestry 50 million years ago which “knocked out” the final enzyme in the hepatic synthesis of vitamin C. The missing enzyme is called GLO (gulano lactone oxidase). Primates such as gorillas, chimpanzees and orangutans also share this same GLO mutation and cannot make vitamin C. In adddition all primates share with humans susceptibility to heart disease.
We humans cannot make vitamin C in our liver as all other animals do. We humans had a genetic mutation in our ancestry 50 million years ago which “knocked out” the final enzyme in the hepatic synthesis of vitamin C. The missing enzyme is called GLO (gulano lactone oxidase). Primates such as gorillas, chimpanzees and orangutans also share this same GLO mutation and cannot make vitamin C. In adddition all primates share with humans susceptibility to heart disease.
Above Image Vitamin C, a simple ring structure simikar to glucose, Ascorbate Courtesy of Wikimedia
All Animals Can Make Vitamin C, but the Guinea Pig Can’t
Except for humans and primates, all other animals have the three enzymes in the liver which can synthesize vitamin C from glucose (a simple sugar). One major exception is the guinea pig, which is really a rodent and not a pig, The guinea pig, for some unexplained reason, shares with humans the identical GLO genetic mutation and also lacks the GLO enzyme just like we do. This makes the guinea pig an ideal experimental model for human diseases. By the way, although animals that make vitamin C never get heart disease, the guinea pig which lacks the ability to synthesize vitamin C, also gets heart disease.
Animals That Make Vitamin C, Don’t Get Atherosclerotic Heart Disease
I though this was worth repeating.
(note: here I am referring to atherosclerotic vascular heart disease in animals. Dogs and Cats DO succumb to other common types of heart disease such as cardiomyopathy and heart worm etc.)
Animals That Don’t Make Vitamin C, Do Get Atherosclerotic Heart Disease
This should be starting to become clear now.
Animal Scientific Support for the Pauling Unified Theory
 As mentioned above, guinea pigs are especially well suited to study atherosclerosis because guinea pigs are unable to make their own vitamin C, and in addition, they develop atherosclerotic plaques similar to those found in humans.
As mentioned above, guinea pigs are especially well suited to study atherosclerosis because guinea pigs are unable to make their own vitamin C, and in addition, they develop atherosclerotic plaques similar to those found in humans.
Left Image Guinea Pig Courtesy of Wikimedia
G. C. Willis, a Canadian doctor, conducted research with guinea pigs in the 1950’s showing that guinea pigs deprived of dietary vitamin C developed atherosclerotic plaques, while guinea pigs given plentiful vitamin C were protected. In addition,guinea pigs fed a vitamin C deficient diet had elevated Lipoprotein (a) levels along with the increased atherosclerotic plaque formation in the arteries.(link)(link)
Similar findings were demonstrated in genetically engineered mice lacking the GLO enzyme. The GLO deficient mice fed a vitamin C deficient diet developed atherosclerotic plaques in the aorta with characteristic deranged collagen crosslinking. GLO deficient mice fed vitamin C were protected. (link)
Human Studies Suporting the Linus Pauling Theory
Optometrist, Dr. Sydney Bush’s retinal artery observations support the Pauling theory. Using modern equipment to non-invasively photograph the retinal arteries of the eye before and after Vitamin C supplementation in humans, Dr. Bush has documented reversal of atherosclerotic plaque with Vitamin C supplementation.(link)
Coronary Calcium Score studies also support the Pauling Theory with favorable results after treatment with Vitamin C and Lysine. (link)
Anecdotal case evidence of reversal of heart disease can be found in the medical literature and documented in Owen Fonorow’s book (see image below). In these cases, advanced heart disease regresses after supplemention with the Linus Pauling Protocol. This is highly significant because the natural course of heart disease is progression, and any intervetion that alters the natural course of a diseae process is highly significant.
Message boards are a source of anecdotal cases reports supporting the Linus Pauling Protocol. The Track Your Plaque Message Boards have documented many cases of regression of atherosclerotic disease with protocols which include vitamin C as well as other nutritional supplements. (There is a modest membership fee for TYP)
Why no drug company sponsored double blind placebo controlled studies? Since there are no patents involved for natural supplements, and no drugs involved, no drug company would ever invest the 250 million dollars to fund such a study with no potential for financial return.
The Physician’s Health Study – Don’t Waste Your Money On Vitamins !!
Using public funds, the NIH (National Institute of Health) funded a large study called The Physicians’ Health Study II which evaluated Vitamins C and E in heart disease and was published Nov. 9, 2008 in JAMA by Howard D. Sesso. The study found that Vitamin C and E did not prevent mortality from heart disease, results which are completely opposite to massive previously published research and anecdotal case reports.
A closer look shows a few glaring errors in study design. The Linus Pauliing Protocol was not followed. The dosage of vitamin C was set too low, at one tenth the dosage recommended by the Linus Pauling protocol, and lysine was not provided. While the Sesso study showed no mortality benefit, many previous studies such as the Enstrom Study showed a striking 40% reduction in all-cause mortality over 6 years from the same 500 mg daily vitamin C dosage. The Paul Knecht study showed a 25% reduction in Heart Disease risk with daily 700 mg Vitamin C. Two previous studies from Japan and Finland showed that Vitamin C reduces risk for stroke, another atherosclerotic disease. There are many more like these.
Since favorable results would be financially destructive to the drug and hospital industries, a cynic might suggest that vested interests were at work in Sesso’s study intending to discredit vitamin C (as documented many times in the past with examples of corporate influence in medical research). It is not difficult to design a medical study to fail. I regard this as merely another example of the information war waged by corporate mainstream medicine against natural medicine.
Enstrom Study, 500 mg Vitamin C Reduces Mortality 40% over 6 years
Enstrom showed that increasing vitamin C intake had a dramatic 40% reduction in mortality benefit which exceeds any statin drug study ever conducted.
Linus Pauling Protocol For Prevention and Reversal of Plaque
If heart disease is chronic scurvy, caused by chronic vitamin C deficiency, then it makes sense to supplement with vitamin C in the amounts needed to make strong collagen and prevent arterial damage from mechanical stress.
In addition, Pauling devised a clever yet simple method to address the issue of Lipoprotein (a) attaching to the lysine residues on the damaged collagen fibers in the arterial wall. He recommended supplementing with 2-4 grams of lysine per day. The additional lysine in the blood stream attaches to the receptor sites on the lipoprotein (a) molecules, inactivating the lipoprotein(a) and preventing it from attaching to the arterial wall. This prevents the initiation of the atherosclerotic process. In addition, Vitamin C and Lysine are both important precursors for building strong collagen which makes strong arteries.
In addition to Lysine, some of the collagen crosslinking is done with another amino acid called Proline, so proline was also added to the treatment protocol.
Where to Read About the Linus Pauling Protocol:

Above image, Cover of Linus Pauling Protocol Book courtesy of Owen Fonorow.
Read more about the Linus Pauling Protocol in this new book (above image): Practicing Medicine Without a License by Owen Fonorow.(link)(link)
Owen Fonorow is perhaps the single most dedicated person devoted to the 1992 Linus Pauling Protocol for prevention and reversal of heart disease.(link) A patent for the Linus Pauling Protocol was issued in 1994.(link) In 1996, a CAT scan coronary calcium score study validated the protocol showing a 15% reversal of calcification indicating reversal of coronary artery disease.(link) Another excellent book on the subject of Vitamin C and heart disease is, Stop America’s #1 Killer by Thomas Levy MD, JD. 2006 (link). To read more on the Linus Pauling Protocol, see this short four page booklet by Owen Fonorow.(link) Or, read this excellent article by English and Cass.(link)
What is the Linus Pauling Protocol?
L-ascorbate (Vitamin C) 5-6 grams a day in divided doses
L-Lysine 5 grams a day in divided doses
L-Proline 2-3 grams a day in divided doses
These supplements can be obtained at any the health food store as tablets or capsules for 40 to 50 dollars a month.
Tower Labs Ascorcine 9
A convenient powder containing all the combined ingredients for the Linus Pauling Protocol is called Ascorsine 9 and can be purchased at Tower Laboratories. This is a good choice for anyone serious about preventing heart disease looking for an easy product with everything in it.
Vitamin E, Good or Bad, Yes or No, Blessing or Curse?
Steve Hickey and Hilary Roberts come right out on page 167 of their book, and make the statement, “Vitamin C and Tocotrienols can reverse coronary artery disease”. They would add the Tocotrienol form of Vitamin E to the Linus Pauling Protocol. Regarding heart disease, and atherosclerotic vascular disease, the authors state that “on the available evidence, the combination of Vitamin C and Tocotrienols could be curative with no known harmful effects.” For a more complete discussion of Vitamin E, see my article, Vitamin E, Curse or Blessing? by Jeffrey Dach MD.
Opposition to the Linus Pauling Protocol by Mainstream Medicine
If you are facing the prospects of coronary artery bypass surgery (left image), you might ask the obvious question: Why hasn’t my cardiologist told me about this information and started me on the Linus Pauling Protocol?
Most cardiologists either don’t know about it or ignore it because of the information war going on between mainstream medicine and natural medicine. Cardiologists read medical journals which regularly run incorrect and biased articles saying Vitamin C is useless for prevention and reversal of heart disease, such as the Nov 9 2008 Sesso study. (link).
Above image: coronary artery bypass operation courtesy wikimedia.
Diagnosing and treating heart disease with expensive tests and procedures such as coronary angiography, angioplasty, stenting and bypass operations is the most profitable part of hospital big business. That’s why hospitals compete and fight with each other over the rights to expand and build larger cardiac cath labs and cardiac bypass operation programs. These programs are huge money makers for the national hospital system.
What would happen if there was a cheap and effective way to reverse and prevent heart disease, (ie. the Linus Pauling Protocol)? Heart disease would become an uncommon illness. With fewer heart patients to treat, the multi-million dollar cath labs and cardiac bypass programs at your local hospital would become obsolete and disappear. This would be a financial catastrophe for mainstream medicine.
With so much money and vested interest at stake, you can imagine why it is not prudent for a cardiologist to bring up the benefits of the Linus Pauling Protocol in friendly conversation while lunching in the Doctor’s Dining Hall. That would be an instant ticket off the medical staff roster and out the hospital door, and the end of a lucrative cardiology practice. What cardiologist in their right mind would do that? It is easier for the mainstream cardiologists to simply laugh it off as a joke and go back to the cath lab, do more procedures and make some money to pay their bills.
Above left image: Hospital expansion project for new cardiac cath lab and cardiac surgery theater. Courtesy wikimedia.
Related articles:
Preventing Heart Attacks with Ouabain
Reversing Heart Disease by Jeffrey Dach MD (Part One)
Heart Disease Part Two by Jeffrey Dach MD
Cholesterol Lowering Statin Drugs for Women, Just Say No by Jeffrey Dach MD
Lipitor and The Dracula of Modern Technology by Jeffrey Dach MD
Vitamin C and Stroke Prevention by Jeffrey Dach MD
Financial Disclosure: I have no financial interest in Vitamin C Foundation, Tower Labororatories or LivOn Labs, Track Your Plaque, nor do I receive income from the sale of any books mentioned here.
Link to this article:http://wp.me/P3gFbV-qL
Jeffrey Dach MD
References and Links
Willis Studies on Vitamin C Guinea Pig Model and Atherosclerosis
http://www.vitamincfoundation.org/pdfs/WillisGround.pdf
AN EXPERIMENTAL STUDY OF THE INTIMAL GROUND SUBSTANCE IN ATHEROSCLEROSIS, G.C. Willis, Canad. M. A. J. Vol 69, 1953, p. 17-22
http://www.vitamincfoundation.org/pdfs/WillisSerial.pdf
SERIAL ARTERIOGRAPHY IN ATHEROSCLEROSIS, G. C. Willis, A. W. Light, W.S. Cow, Canad. M. A. J. Dec 1954, Vol 71, 1954, p. 562-568
http://www.vitamincfoundation.org/pdfs/WillisTissue.pdf
ASCORBIC ACID CONTENT OF HUMAN ARTERIAL TISSUE, G. C. Willis, S. Fishman, Canad. M. A. J., April 1, 1955, Vol 72, Pg 500-503
http://www.vitamincfoundation.org/pdfs/WillisAthero.pdf
THE REVERSIBILITY OF ATHEROSCLEROSIS, G. C. Willis, Canad. M. A. J., July 15, 1957, Voll 77., Pg 106-109
http://www.vitamincfoundation.org/pdfs/
CAPILLARY RUPTURE WITH INTIMAL HEMORRHAGE IN THE CAUSATION OF CEREBRAL VASCULAR LESIONS, J. C. Paterson, Arch Path, Vol 29, 1940, Pg 345-354
http://www.vitamincfoundation.org/pdfs/
SOME FACTORS IN THE CAUSATION OF INTIMAL HEMORRHAGES AND IN THE PRECIPITATION OF CORONARY THROMBI, J. C. Paterson, Canad. M. A. J., Feb 1941, Pg 114-120
Guinea Pigs are excellent model for atherosclerosis
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1435897
Nutr Metab (Lond). 2006; 3: 17.
Guinea pigs: A suitable animal model to study lipoprotein metabolism, atherosclerosis and inflammation by Maria Luz Fernandez1 and Jeff S Volek2
http://jn.nutrition.org/cgi/content/full/131/1/10
Journal of Nutrition. 2001;131:10-20.
Guinea Pigs as Models for Cholesterol and Lipoprotein Metabolism Maria Luz
http://ci.nii.ac.jp/naid/110002603577/
Japanese circulation journal Vol.35, No.12(19711200) pp. 1559-1565
EXPERIMENTAL ATHEROSCLEROSIS WITH ASCORBIC ACID DEFICIENCY by FUJITANI TAKAO et al.
Influence of ascorbic acid deficiency on serum and hepatic lipid and on the aorta were studied. Two groups of ascorbic acid deficient guinea pigs were made by feeding with scorbutic diet with or without 5% coconut oil for two weeks (group 3 and 1).
Control animals of the experimental groups were fed with the same diet of previous two groups and received 25 mg of ascorbic acid subcutaneously for the same period (group 2 and 4).
Moderate increase of triglyceride and cholesterol ester and beta-lipoprotein in the serum of ascorbic acid deficiency (group 1), and markedly to approximately twice of normal in the ascorbic acid deficiency with coconut oil feeding (group 3).
Increase of serum lipids and depression of plasma lipoprotein lipase activity by ascorbic acid deficiency was prevented by ascorbic acid administration as observed in the control groups. Histological examination of the aorta revealed edematous swelling of the ground substance in the intima and media in the scorbutic, and early atheromatous lesions of accumulated foam cells in the intima of the ascorbic acid deficiency with coconut oil feeding. These findings suggest that any factors disturbing ascorbic acid metabolism induce an increase of serum lipids and altered vascular wall metabolism, and consequently follows atherosclerosis.
Landmark Linus Pauling Articles
http://www.pnas.org/content/87/23/9388.full.pdf
Immunological evidence for the accumulation of lipoprotein(a) in the atherosclerotic lesion of the hypoascorbemic guinea pig. M Rath, L Pauling – Proceedings of the National Academy of Sciences, 1990 – National Acad Sciences. Vol. 87, pp. 9388-9390, December 1990
http://www.pnas.org/content/87/16/6204.full.pdf
Hypothesis: lipoprotein (a) is a surrogate for ascorbate.
M Rath, L Pauling – Proceedings of the National Academy of Sciences 1990
Another link to same article:
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=2143582
Proc Natl Acad Sci U S A. 1990 August; 87(16): 6204–6207. PMCID: PMC54501
Hypothesis: lipoprotein(a) is a surrogate for ascorbate. M Rath and L Pauling
http://faculty.washington.edu/ely/paulinglysine.html
Journal of Orthomolecular Medicine, 6(3-4): 144-46, 1991.
http://www.orthomed.org/jom/jom.htm
Case Report: Lysine/Ascorbate-Related Amelioration of Angina Pectoris by Linus Pauling
http://orthomolecular.org/library/jom/1992/pdf/1992-v07n01-p005.pdf
A Unified Theory of Human Cardiovascular Disease Leading the Way to the Abolition of This Disease as a Cause for Human Mortality M Rath, L Pauling – J Ortho Med, 1992 –
http://www.americanhearthealthservices.com/images/Cellular_Essentials.pdf
JOURNAL OF APPLIED NUTRITION, VOLUME 48, NUMBER 3, 1996 ORIGINAL REPORT
Copyright – International Academy of Nutrition and Preventive Medicine
NUTRITIONAL SUPPLEMENT PROGRAM HALTS PROGRESSION OF EARLY CORONARY ATHEROSCLEROSIS DOCUMENTED BY ULTRAFAST COMPUTED TOMOGRAPHY
Matthias Rath, M.D. and Aleksandra Niedzwiecki, Ph.D.
Other supportive articles
http://www.ncbi.nlm.nih.gov/pubmed/1588941
Mol Cell Biochem. 1992 Apr;111(1-2):41-7.
Protective role of ascorbic acid against lipid peroxidation and myocardial injury.
Chakrabarty S, Nandi A, Mukhopadhyay CK, Chatterjee IB. Department of Biochemistry, University College of Science, Calcutta, India.
Ascorbic acid (AH2) is a potential scavenger of superoxide radical and singlet oxygen. In the guinea pig, marginal AH2 deficiency results in intracellular oxidative damage in the cardiac tissue as evidenced by lipid peroxidation, formation of fluorescent pigment and loss of structural integrity of the microsomal membranes. The oxidative damage does not occur due to lack of enzymatic scavengers of reactive oxygen species such as superoxide dismutase, catalase and glutathione peroxidase. Also, glutathione transferase activity is not decreased in AH2 deficiency. Lipid peroxidation, fluorescent pigment formation and protein modification disappear after AH2 therapy. These results, if extra-polated to human beings, would indicate that chronic subclinical AH2 (ascorbate) deficiency may result in progressive oxidative damage which in the long run may lead to permanent degenerative diseases in the heart.
High Blood sugar worsens effect of vitamin C Deficiency
http://www.ncbi.nlm.nih.gov/pubmed/11500168
Atherosclerosis. 2001 Sep;158(1):1-12.
Hyperglycemia-induced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis.Price KD, Price CS, Reynolds RD.
Dehydroascorbic acid, the oxidized form of vitamin C, is transported into mammalian cells via facilitative glucose transporters and hyperglycemia inhibits this process by competitive inhibition. This inhibited transport may promote oxidative stress and contribute to the increase in atherosclerotic cardiovascular disease observed in patients with diabetes mellitus.
http://www.ajcn.org/cgi/reprint/23/1/27.pdf
Am J Clin Nutr. 1970 Jan;23(1):27-30.
Ascorbic acid and atherosclerosis.Shaffer CF.
Anti-Vitamin C Articles, Failure of Vitamin C
http://jama.ama-assn.org/cgi/content/full/2008.600
Vitamins E and C in the Prevention of Cardiovascular Disease in Men
The Physicians’ Health Study II Randomized Controlled Trial – JAMA-EXPRESS
Howard D. Sesso, ScD, MPH; Julie E. Buring, ScD; William G. Christen, ScD; Tobias Kurth, MD, ScD; Charlene Belanger, MA; Jean MacFadyen, BA; Vadim Bubes, PhD; JoAnn E. Manson, MD, DrPH; Robert J. Glynn, ScD; J. Michael Gaziano, MD, MPH
JAMA. 2008;300(18):2123-2133. Published online November 9, 2008 (doi: 10.1001/jama.2008.600).
Basic research and observational studies suggest vitamin E or vitamin C may reduce the risk of cardiovascular disease. However, few long-term trials have evaluated men at initially low risk of cardiovascular disease, and no previous trial in men has examined vitamin C alone in the prevention of cardiovascular disease.
Objective To evaluate whether long-term vitamin E or vitamin C supplementation decreases the risk of major cardiovascular events among men.
Design, Setting, and Participants The Physicians’ Health Study II was a randomized, double-blind, placebo-controlled factorial trial of vitamin E and vitamin C that began in 1997 and continued until its scheduled completion on August 31, 2007. There were 14 641 US male physicians enrolled, who were initially aged 50 years or older, including 754 men (5.1%) with prevalent cardiovascular disease at randomization.
Intervention Individual supplements of 400 IU of vitamin E every other day and 500 mg of vitamin C daily.
Main Outcome Measures A composite end point of major cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, and cardiovascular disease death).
Results During a mean follow-up of 8 years, there were 1245 confirmed major cardiovascular events. Compared with placebo, vitamin E had no effect on the incidence of major cardiovascular events (both active and placebo vitamin E groups, 10.9 events per 1000 person-years; hazard ratio [HR], 1.01 [95% confidence interval {CI}, 0.90-1.13]; P = .86), as well as total myocardial infarction (HR, 0.90 [95% CI, 0.75-1.07]; P = .22), total stroke (HR, 1.07 [95% CI, 0.89-1.29]; P = .45), and cardiovascular mortality (HR, 1.07 [95% CI, 0.90-1.28]; P = .43). There also was no significant effect of vitamin C on major cardiovascular events (active and placebo vitamin E groups, 10.8 and 10.9 events per 1000 person-years, respectively; HR, 0.99 [95% CI, 0.89-1.11]; P = .91), as well as total myocardial infarction (HR, 1.04 [95% CI, 0.87-1.24]; P = .65), total stroke (HR, 0.89 [95% CI, 0.74-1.07]; P = .21), and cardiovascular mortality (HR, 1.02 [95% CI, 0.85-1.21]; P = .86). Neither vitamin E (HR, 1.07 [95% CI, 0.97-1.18]; P = .15) nor vitamin C (HR, 1.07 [95% CI, 0.97-1.18]; P = .16) had a significant effect on total mortality but vitamin E was associated with an increased risk of hemorrhagic stroke (HR, 1.74 [95% CI, 1.04-2.91]; P = .04).
Conclusions In this large, long-term trial of male physicians, neither vitamin E nor vitamin C supplementation reduced the risk of major cardiovascular events. These data provide no support for the use of these supplements for the prevention of cardiovascular disease in middle-aged and older men.
Trial Registration clinicaltrials.gov Identifier: NCT00270647
http://circ.ahajournals.org/cgi/content/full/105/12/1396
Vitamin C, Collagen, and Cracks in the Plaque, by Peter Libby, MD; Masanori Aikawa, MD PhD
Most fatal acute myocardial infarctions result from a fracture of the plaque’s fibrous cap. We proposed the hypothesis some years ago that the level of collagen in the fibrous cap depends on a dynamic balance of synthesis and degradation.1 We further showed that inflammatory cytokines can regulate both the expression of genes that direct interstitial collagen synthesis in vascular smooth muscle cells and the interstitial collagenases (matrix metalloproteinases 1, 8, and 13) required to initiate the breakdown of collagen fibrils.2–5 Given the capital importance of collagen in protecting the plaque from rupture and hence thrombosis, the metabolism of this complex molecule merits consideration in depth. See p 1485
The formation of mature fibrillar collagen involves many steps beyond gene transcription (Figure). The initial translation product, the procollagen peptide chain, undergoes extensive posttranslational modification: an especially noteworthy point during this period of exploration of the proteome. Collagen contains unusual amino acids, hydroxyproline and hydroxylysine, formed by a vitamin C–dependent process that entails enzymatic transfer of hydroxyl groups to selected proline and lysine residues in the nascent procollagen chains. Glycosyl transferases then add sugar moieties to the procollagen chains. The hydroxylated and glycosylated monomers then self-assemble into helical trimers as they traverse several intracellular compartments. Trimming the nonhelical tails (the telopeptides) from both ends of the procollagen molecule by proteinases yields the mature interstitial collagen triple helix secreted by smooth muscle cells in arteries. These building blocks then further self-aggregate into multimers and form interstitial collagen fibrils, linear structures as strong as steel wires.
The ascorbate-dependent addition of polar hydroxyl groups to the side chains of proline and lysine may aid the self-assembly and stability of the collagen fibril by forming interchain hydrogen bonds. The absence of sufficient ascorbic acid (vitamin C), a required cofactor for prolylhydroxylase, thus impairs the formation of stable collagen. The human phenotype of vitamin C deficiency, scurvy, classically involves fragility of blood vessels. In 1753, James Lind described the skin of scorbutics as “…covered with several reddish, bluish… spots… resembling an effusion of blood.”6 Lind’s discovery that food rich in vitamin C provided a cure for scurvy spurred England’s subsequent naval supremacy and putatively changed the course of history. Of course, chemical characterization of vitamin C as the antiscorbutic factor did not occur until the 1930s. (Interested readers may find Albert Szent-Györgi’s dispute with the editor of the Biochemical Journal regarding publication of the structure of vitamin C amusing.7 Cardiologists know Szent-Györgi better for his later discovery of the biochemical basis of muscle contraction).
Study of vitamin C’s role in vivo has proven challenging. Unlike humans, usual experimental animals can synthesize vitamin C and thus cannot be made scorbutic. Maeda’s group has recently introduced a targeted mutation that makes mice dependent on dietary vitamin C, allowing manipulation of ascorbate levels. In this issue of Circulation, Nakata et al8 studied atheroma in hypercholesterolemic and scorbutic mice. They find no change in lesion size, but they observe decreased collagen content in the lesions. Such changes should impair the biomechanical strength of the plaque and make it more prone to rupture. This finding extends the burgeoning evidence that changes in collagen metabolism can influence crucial characteristics of the atherosclerotic plaque. Our recent in vivo studies also showed that alterations in collagen synthesis and catabolism induced by lipid lowering or statin treatment influence the collagenous structure of atheroma in rabbits.9–11 It would have been of interest to evaluate overall protein content in these lesions, as we showed almost 2 decades ago that ascorbate can augment noncollagen protein synthesis by cultured arterial smooth muscle cells.12
We do not know whether scorbutic humans with atherosclerosis would experience increased plaque rupture, a curious but currently clinically irrelevant question. In addition to its antiscorbutic action, vitamin C has potent antioxidant properties. Physiological concentrations of ascorbic acid can inhibit in vitro oxidative modification of LDL, a critical event during atherogenesis.13 For this reason, many individuals take this and other antioxidant vitamins in hope that combating oxidative stress can forestall atherosclerosis and its complications. Vitamin C has other antiinflammatory effects as well, including decreased leukocyte adhesion to the endothelium and increased bioavailability of atheroprotective nitric oxide (NO). Administration of vitamin C for 10 days (2 g/d) reduces adhesion of monocytes obtained from cigarette smokers to cultured endothelial cells.14 Vitamin C’s potency generally exceeds that of vitamin E as an antiinflammatory and antiatherogenic agent. For example, intake of vitamin C, but not vitamin E, inhibits oxidized LDL-induced leukocyte adhesion to endothelium in hamsters.15 Physiological concentrations of ascorbic acid also increase synthesis and activity of NO in vitro.16 Vitamin C also inhibits activation of nuclear factor-B (NF-![]() , a key regulator of inflammatory gene expression.17 Administration of vitamin C can improve endothelial dysfunction in hypercholesterolemic patients.18 Ascorbate’s effect on plaque collagen content adds another theoretical rationale for using vitamin C in patients at risk for atherosclerotic events.
, a key regulator of inflammatory gene expression.17 Administration of vitamin C can improve endothelial dysfunction in hypercholesterolemic patients.18 Ascorbate’s effect on plaque collagen content adds another theoretical rationale for using vitamin C in patients at risk for atherosclerotic events.
Unfortunately, we lack clinical evidence that would permit us to translate this basic science into practice. The recently reported (but as yet unpublished) Heart Protection Study (HPS) administered a cocktail of antioxidant vitamins (vitamin C 250 mg, vitamin E 600 mg, beta-carotene 20 mg/d) to a large group of individuals at high risk for atherosclerotic events for a period of 5 years. The vitamins had absolutely no effect on a variety of end points, including coronary events 19 (see also The Heart Protection Study Investigators at http://www.hpsinfo.org/). Brown and colleagues recently reported acceleration of coronary atherosclerosis in patients treated with a combination of statins and a cocktail of antioxidant vitamins (vitamin C 1000 mg, vitamin E 800 IU, beta-carotene 25 mg/d) in the HDL-Atherosclerosis Treatment Study (HATS).20
To what may we attribute this apparent failure of a plausible and widely used therapeutic approach? First, these studies may have employed suboptimal doses of the vitamins used, or interactions among them may have mitigated a beneficial effect of one or the other supplements. In this regard, vitamin E monotherapy showed no benefit on atherosclerotic events in both the Heart Outcomes Prevention Evaluation (HOPE) and GISSI studies.21,22 In these various studies, the degree of preexisting disease or inadequate duration of treatment might have limited the benefit of the antioxidants. Yet, the striking beneficial effects observed in the statin treatment arms of both the HPS and HATS20 and of the angiotensin converting enzyme inhibitor in the HOPE study21 establish the mutability of outcomes measured in these patient populations in the same trials. Partition of fat-soluble vitamin E into the lipid phase of plaque or lipoproteins might shield it from the cooperative antioxidant effect of water-soluble ascorbate, excluded from these lipid milieux. Thus, more potent or amphipathic antioxidants might interrupt oxidative stress during atherogenesis more effectively than the vitamins. Although vitamin deficiencies lead to disease, consumption of pharmacological amounts of these substances in individuals who maintain vitamin-sufficient diets may not prevent disease. Indeed, malnutrition hardly applies to our patients with atherosclerosis. Contemporary developed societies seem at much higher risk of dietary surfeit than lack.
It is curious indeed that many remain suspicious of pharmacological lipid-lowering, a strategy now proven unequivocally to prevent myocardial infarction and stroke and to prolong life as well. Yet, many individuals readily consume costly vitamin supplements devoid of benefit in clinical trials. This situation may reflect in part a failure of the medical community to communicate effectively with the public regarding evidence-based medicine and the life-saving benefits of preventive strategies. The present results of Nakata et al8 reinforce the importance of collagen metabolism in determining the structure of atherosclerotic plaques. However, current clinical and experimental evidence suggests that the best way to influence favorably the balance of collagen synthesis and degradation in atheroma at hand today remains lipid-lowering, not vitamin C.
Enstrom Study 500 mg Vitamin C Reduces Mortality 40% over 6 years
http://www.ncbi.nlm.nih.gov/pubmed/1591317
Epidemiology. 1992 May;3(3):194-202
Vitamin C intake and mortality among a sample of the United States population.Enstrom JE, Kanim LE, Klein MA.School of Public Health, University of California, Los Angeles 90024.
We examined the relation between vitamin C intake and mortality in the First National Health and Nutrition Examination Survey (NHANES I) Epidemiologic Follow-up Study cohort. This cohort is based on a representative sample of 11,348 noninstitutionalized U.S. adults age 25-74 years who were nutritionally examined during 1971-1974 and followed up for mortality (1,809 deaths) through 1984, a median of 10 years. An index of vitamin C intake has been formed from detailed dietary measurements and use of vitamin supplements. The relation of the standardized mortality ratio (SMR) for all causes of death to increasing vitamin C intake is strongly inverse for males and weakly inverse for females.
Among those with the highest vitamin C intake, males have an SMR (95% confidence interval) of 0.65 (0.52-0.80) for all causes, 0.78 (0.50-1.17) for all cancers, and 0.58 (0.41-0.78) for all cardiovascular diseases; females have an SMR of 0.90 (0.74-1.09) for all causes, 0.86 (0.55-1.27) for all cancers, and 0.75 (0.55-0.99) for all cardiovascular diseases.
Comparisons are made relative to all U.S. whites, for whom the SMR is defined to be 1.00. There is no clear relation for individual cancer sites, except possibly an inverse relation for esophagus and stomach cancer among males. The relation with all causes of death among males remains after adjustment for age, sex, and 10 potentially confounding variables (including cigarette smoking, education, race, and disease history).
25% reduction in Heart Disease Risk with 700 mg Vit C
http://www.ajcn.org/cgi/content/full/80/6/1508
American Journal of Clinical Nutrition, Vol. 80, No. 6, 1508-1520, December 2004
Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts
Paul Knekt et al.
Epidemiologic studies have suggested a lower risk of coronary heart disease (CHD) at higher intakes of fruit, vegetables, and whole grain. Whether this association is due to antioxidant vitamins or some other factors remains unclear.
Objective:We studied the relation between the intake of antioxidant vitamins and CHD risk.
Design:A cohort study pooling 9 prospective studies that included information on intakes of vitamin E, carotenoids, and vitamin C and that met specific criteria was carried out. During a 10-y follow-up, 4647 major incident CHD events occurred in 293 172 subjects who were free of CHD at baseline.
Results![]() ietary intake of antioxidant vitamins was only weakly related to a reduced CHD risk after adjustment for potential nondietary and dietary confounding factors. Compared with subjects in the lowest dietary intake quintiles for vitamins E and C, those in the highest intake quintiles had relative risks of CHD incidence of 0.84 (95% CI: 0.71, 1.00; P = 0.17) and 1.23 (1.04, 1.45; P = 0.07), respectively, and the relative risks for subjects in the highest intake quintiles for the various carotenoids varied from 0.90 to 0.99. Subjects with higher supplemental vitamin C intake had a lower CHD incidence. Compared with subjects who did not take supplemental vitamin C, those who took >700 mg supplemental vitamin C/d had a relative risk of CHD incidence of 0.75 (0.60, 0.93; P for trend < 0.001).
ietary intake of antioxidant vitamins was only weakly related to a reduced CHD risk after adjustment for potential nondietary and dietary confounding factors. Compared with subjects in the lowest dietary intake quintiles for vitamins E and C, those in the highest intake quintiles had relative risks of CHD incidence of 0.84 (95% CI: 0.71, 1.00; P = 0.17) and 1.23 (1.04, 1.45; P = 0.07), respectively, and the relative risks for subjects in the highest intake quintiles for the various carotenoids varied from 0.90 to 0.99. Subjects with higher supplemental vitamin C intake had a lower CHD incidence. Compared with subjects who did not take supplemental vitamin C, those who took >700 mg supplemental vitamin C/d had a relative risk of CHD incidence of 0.75 (0.60, 0.93; P for trend < 0.001).
Conclusions:The results suggest a reduced incidence of major CHD events at high supplemental vitamin C intakes.
Vitamin C Deficiency In the US
http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=15117714
Am J Public Health. 2004 May; 94(5): 870–875. PMCID: PMC1448351
Vitamin C Deficiency and Depletion in the United States: The Third National Health and Nutrition Examination Survey, 1988 to 1994 Jeffrey S Hampl, et al. In conclusion, our data indicate that a considerable number of children and adults in the United States are vitamin C deficient or depleted.
Owen Fonorow Articles on Vitamin C
http://www.internetwks.com/owen/TheoryPaper.htm
The Unified Theory, The Long Neglected Theory of Cardiovascular and Heart Disease By Owen Richard Fonorow 2002
1) CV Mortality declines after Vitamin C Consumption increases after publication of Linus PAuling Book published 1970. US is only industrialized country to experience a 40% drop in CV disease.
2) Most high order mammals make their own vitamin C (3-11 g daily) do not have CV (cardiovascular) disease as seen in humans.
3) Animals such as the Guinea pig which cannot make their own vitamin C, also have cardiovascular disease, same as humans if they are fed a Vitamin C deficient diet.
4) The Willis Guinea Pig Experiments in the 1950’s show that depriving the animal of vitamin C causes atherosclerosis which is quite similar to the human lesion. No plaque forms in the control group getting “adequate” vitamin C. (Source: The Reversibility of Atherosclerosis, G. C. Willis, Canad M. A. J, July 15, 1957, Vol 77)
5) Pauling and Rath showed the apo(a)/Lp(a) increased in the animals deprived of vitamin C, but not in the controls. (Source: Immunological evidence for the accumulation of Lp(a) in the atherosclerotic lesion of the hypoascorbemic guinea pig, Pauling/Rath, Pro. Nat. Acad. Sci USA, Vol 87, pp 9388-9390, Dec 1990, Biochem)
6) The heart disease process begins with a lesion in the artery. (Source: Brown-Goldstein Scientific American 1982, and Linus Pauling Heart Disease Video)
7) Veins, in general, do not suffer plaque deposits. (Source: Common knowledge from the medical Literature)
8) Plaques are often localized to areas of mechanical stress, at bifurcations, and the surface of the heart (coronary arteries or carotid arteries). (Source: An Experimental Study of the Intimal Ground Substance in Atherosclerosis, G. C. Willis, Canad M. A. J., July 1953, Vol 69, pg 17)
9) Vitamin C is required (and used up) making collagen – the most abundant protein in the human body. (Source: Roger J. Williams, Nutrition Against Disease, 1971, Linus Pauling HOW TO LIVE LONGER AND FEEL BETTER, 1986)
10) Scurvy is caused by a deficiency in making collagen which is in turn caused by a lack of vitamin C. (Source: James Lind, 1753, Linus Pauling HOW TO LIVE LONGER AND FEEL BETTER 1986)
11) The Beisiegel studies in Germany of post-mortem human aortas in 1989 determined that plaque consists of Lp(a) and only Lp(a) – no ordinary LDL. (Source: Morphological detection and quantification of lipoprotein(a) deposition in atheromatous lesions of human aorta and coronary arteries in Virchows Arch A Pathol Anat Histopathol 1990;417(2):105-11, Niendorf A; Dietel M; Beisiegel U; Arps H; Peters S , Wolf K; Rath M and Lipoprotein(a) in the arterial wall. Beisiegel U; Rath M; Reblin T; Wolf K; Niendorf A, Eur Heart J 1990 Aug;11 Suppl E:174-83)
12) Elevated cholesterol has been correlated with CVD, but many studies were conducted before Lp(a) was known, Lp(a) was lumped in with LDL. In September, 2000, an Oxford meta-analysis of 27 large studies showed that people with elevated Lp(a) are 70% more like to suffer a heart attack or stroke. (Source: Circulation Sep 2000)
Radio Interviews
mms://rense.gsradio.net/rense/windows_media/
WMHigh/May2008/kx2u91/rense_052208_hr2.wma
Owen Fonorow First Interview MAy 2008 on Jeff Rense
1/2 to 1 million cardiovascular deaths per year can be prevented with vitamin C. There are about a million cardiac procedures (angioplasty, stent, bypass) per year. We have a genetic defect in the inability to make vitamin C. We have a defect in GLO enzyme which happened 50 million years ago. High level primates, guineas pig and fruit bat cannot make vitamin C. People with angina and chest pain usually get better in 10 days on Vitamin C and Lysine. WHen they stop the supplements, they may have a recurrence of pain. Case report of a patient: Unique E and High Vitamin C (6 grams), Lysine (6 grams) reverts EKG back to normal. About 6 months after stopping, heart problems recurr. 50 million years ago, Lipoprotein (a) evolved with Lysine binding site to allow us to evolve. Lipoportein (a) forms cholesterol plaque in absence of Vitamin C. Medical Study: Examination of aortas, lipoprotein (a) found in plaque. Most people live with chronic low levels of vitamin C. Cardio-C drink mix with Pauling recommendation. Contains 2-3 grams of Lysine, 3 grams of Vitamin C. After 12 years of experience: it does reverse atherosclerosis which is a symptom of chronic scurvy which builds plaque in the arteries.
(Bush) White atheromas can be seen in retinal arteries. In the Vitamin C group, these plaques reverse, This work was published. With 10 grams of Vitamin C a day, and it takes a month to reverse soft atheromas. Calcified atheromas takes longer. Once case took 2 years to reverse.Cardio-Retinometry: We see amazing reversals in a very short time. Not noticed by cardiologists.Vitamin C is safe with no adverse effects. Therapeutic Dosage: take 500 mg-1000mg Vitamin C every 4 hours. Non-Chinese Vitamin C made in Europe Vitamin C Foundation.
Cost is : 24 dollars a month auto-ship Cardio C. Liposomal Vitamin C is available for people with gas and diarrhea. More expensive.
Second Radio Interview
mms://rense.gsradio.net/rense/windows_media/WMHigh/Aug2008/r8r10x/
rense_081408_hr2.wma
Radio Interview on Vitamin C and Heart Disease with Owen Fonorow Aug 2008
Heart Disease and Vitamin C, Linus Pauling Genius discovered a way to stop and reverse heart disease in humans. Owen Fonorow, Vitmain C Foundation. So simple, yet so crucial. Remarkable breakthrough in human health and well ness. Book: Practicing Medicine Without A License, the story of Linus Pauling and Heart Disease by Owen Fonorow. Carol Smith experience relase and recovery on three ocassions. Basic theory is that most of animal species produce own bitamin C in the liver. Humans cannot make vitamin C, and we suffer cardiovascular disease.
The species that make vitamin C can make collagen which keeps artieries strong. In the absence of vitamin C production we have evolved cholesterol plaques to repair the arteries causing occluded arteries and heart attacks. We can no longer produce vitamin C due to a genetic defect. Lysine binding site. Lysine is the second component. High dose, needs powser and avoid pills. Collagen is the major protein of connective tissue and arteries. Lack of vitamin C causes weak collagen.
http://www.internetwks.com/owen/
Health Articles by Owen Fonorow, Orthomolecular Naturopath (Orthomopath)
(c) 1996-2008 Owen Fonorow.
http://practicingmedicinewithoutalicense.com/protocol/excerpt_chp7.pdf
Chapter 7 Practicing Medicine WIthout a License summary of Pauling therapy to reverse heart disease
http://www.vitamincfoundation.org/ Vitamin C Foundation, Owen Fonorow
Article Summarizing Linus Pauling Theory of Heart DIsease
http://www.nutritionreview.org/library/collagen.connection.html
Linus Pauling’s Unified Theory of Human Cardiovascular Disease
The Collagen Connection Jim English and Hyla Cass, MD
Pauling Therapy for the Reversal of Heart Disease
1. Vitamin C: to bowel tolerance – as much as you can take without diarrhea. For most people this will be in the range of five to ten grams (5,000-10,000 mg.) each day. Spread this amount into two equal doses 12 hours apart. (Vitamin C prevents further cracking of the blood vessel wall – the beginning of the disease.)
2. L-Proline: 3 grams twice per day (acts to release lipoprotein(a) from plaque formation and prevent further deposition of same).
3. L-Lysine: 3 grams twice each day (acts to release lipoprotein(a) from plaque formation and prevent further deposition of same).
4. Co-enzyme Q10: 90-180 mg. twice per day (strengthens the heart muscle).
5. L-Carnitine: 3 grams twice per day (also strengthens the heart muscle).
6. Niacin: Decreases production of lipoprotein(a) in the liver. Inositol hexanicotinate is a form of niacin which gives less of a problem with flushing and therefore allows for larger therapeutic doses. Begin with 250 mg. at lunch, 500 mg. at dinner and 500 mg. at bedtime the first day; then increase gradually over a few days until you reach four grams per day, or the highest dose under four grams you can tolerate. Be sure to ask your doctor for liver enzyme level tests every two months or less to be sure your liver is able to handle the dose you are taking.
7. Vitamin E: 800-2400 IU per day. (Inhibits proliferation of smooth muscle cells in the walls of arteries undergoing the atherosclerotic changes.)
References for Cass article:
1. Marcoux C; Lussier-Cacan S; Davignon J; Cohn JS.
Association of Lp(a) rather than integrally-bound apo(a) with triglyceride-rich lipoproteins of human subjects.
Biochim Biophys Acta 1997 Jun 23;1346(3):261-74.
2. Ensenat D, Hassan S, Reyna SV, Schafer AI, Durante W.
Transforming growth factor-b1 stimulates vascular smooth muscle cell L-proline transport by
inducing system A amino acid transporter 2 (SAT2) gene expression. Biochem. J. (2001) 360, (507-512)
3. White AL; Lanford RE. Cell surface assembly of lipoprotein(a) in primary cultures of baboon hepatocytes.
J Biol Chem 1994 Nov 18;269(46):28716-23.
4. Klezovitch O; Edelstein C; Scanu AM. Evidence that the fibrinogen binding domain of
Apo(a) is outside the lysine binding site of kringle IV-10:
a study involving naturally occurring lysine binding defective lipoprotein(a) phenotypes.
J Clin Invest 1996 Jul 1;98(1):185-91.
5. Boonmark NW; Lou XJ; Yang ZJ; Schwartz K; Zhang JL; Rubin EM; Lawn RM.
Modification of apolipoprotein(a) lysine binding site reduces atherosclerosis in transgenic mice.
J Clin Invest 1997 Aug 1;100(3):558-64.
6. Phillips J; Roberts G; Bolger C; el Baghdady A; Bouchier-Hayes D; Farrell M; Collins P.
Lipoprotein (a): a potential biological marker for unruptured intracranial aneurysms.
Neurosurgery 1997 May;40(5):1112-5; discussion 1115-7.
7. Stubbs P; Seed M; Moseley D; O’Connor B; Collinson P; Noble M.
A prospective study of the role of lipoprotein(a) in the pathogenesis of unstable angina.
Eur Heart J 1997 Apr;18(4):603-7.
8. Shinozaki K; Kambayashi J; Kawasaki T; Uemura Y; Sakon M; Shiba E; Shibuya T; Nakamura T; Mori T.
The long-term effect of eicosapentaenoic acid on serum levels of lipoprotein (a) and lipids in patients with vascular disease. J Atheroscler Thromb 1996;2(2):107-9.
9. McCully KS, Homocysteine metabolism in scurvy, growth and arteriosclerosis. Nature 1971;231:391-392.
10. Pauling L, Rath M. Pro. Nat. Acad. Sci USA, Vol 87, pp 9388-9390, Dec 1990.
11. Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. American Journal of Clinical Nutrition 1999;69(6):1086-1107.
12. Simon JA, Hudes ES. Serum ascorbic acid and gallbladder disease prevalence among US adults: the Third National Health and Nutrition Examination Survey (NHANES III). Arch Intern Med. 2000;160(7):931-936.
13. Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. Journal of Emerg Med. 2001;21(3):235-237.
14. Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci U S A. 1976;73(10):3685-3689.
http://www.orthomolecular.org/library/jom/1992/pdf/1992-v07n03-p153.pdf
[PDF] Reducing the Risk for Cardiovascular Disease with Nutritional Supplements M Rath. Niacin and ascorbate have been reported to lower plasma lipoprotein-a levels. … with the binding of lipoprotein-a to collagen and other matrix components. …
LipoProtein (a) is independent marker of atherosclerosis
http://www.chestjournal.org/cgi/content/abstract/121/5/1589
Chest. 2002;121:1589-1594.
Elevated Serum Lipoprotein(a) Level Is an Independent Marker of Severity of Thoracic Aortic Atherosclerosis Marcel Peltier, MD et al.
Conclusion: This prospective study indicates that serum Lp(a) level is an independent marker of severity of thoracic aortic atherosclerosis detected by multiplane TEE. These findings emphasize the role of Lp(a) as a marker of atherosclerotic lesions in the major arterial locations.
Lipoprtein (a) detected in arterial wall contributing to plaque formation
http://www.ncbi.nlm.nih.gov/pubmed/2528948
Arteriosclerosis. 1989 Sep-Oct;9(5):579-92.Links
Detection and quantification of lipoprotein(a) in the arterial wall of 107 coronary bypass patients. Rath M, Niendorf A, Reblin T, Dietel M, Krebber HJ, Beisiegel U.
Medizinische Kern und Poliklinik, Universitäts-Krankenhaus Eppendorf, Hamburg, FRG.
The aim of this study was to determine the extent of accumulation of lipoprotein(a) [Lp(a)] in human arterial wall and to define its potential role in atherogenesis. Biopsies routinely taken from the ascending aorta of 107 patients undergoing aortocoronary bypass surgery were analyzed for lipid and lipoprotein parameters, which were then correlated to serum values. A significant positive correlation was established between serum Lp(a) and arterial wall apolipoprotein (apo)(a) by enzyme-linked immunosorbent assay. High serum Lp(a) also led to a significant increase of apo B in the arterial wall. No significant correlation was found between apo B in serum and aortic tissue. Apo B was found to be partially linked to apo(a) in the aortic extract. Furthermore, apo(a) was found to be intact, as determined by its molecular weight in sodium dodecyl sulfate electrophoresis. This technique also revealed that the apo(a) isoform pattern of aortic homogenate was comparable to the individual serum pattern. Immunohistochemical methods demonstrated a striking colocalization of apo(a) and apo B in the arterial wall, predominantly located extracellularly. Both proteins were increased in atherosclerotic plaques. With density gradient ultracentrifugation, Lp(a)-like particles could be isolated from plaque tissue. This initial study showed that Lp(a) accumulates in the arterial wall, partly in the form of lipoprotein-like particles, therefore contributing to plaque formation and coronary heart disease.
http://www.ncbi.nlm.nih.gov/pubmed/7605357
Atherosclerosis. 1995 Mar;113(2):179-88.
Extraction of lipoprotein(a), apo B, and apo E from fresh human arterial wall and atherosclerotic plaques.Reblin T, Meyer N, Labeur C, Henne-Bruns D, Beisiegel U. Medizinische Kernklinik und Poliklinik, Universitätskrankenhaus Eppendorf (UKE), Hamburg, Germany.
Several studies have analysed apo(a) quantitatively in arterial wall tissue derived from post mortem samples. The purpose of this study was a qualitative analysis of Lp(a) in fresh human arterial wall tissue. It was evaluated whether Lp(a) exists as an intact lipoprotein or whether it is degraded. Additionally it was analysed whether there are differences in the apolipoprotein composition between lesion-free and diseased human arterial wall tissue. Serum and intimal tissue samples taken from the abdominal aorta and the inferior caval vein of 18 organ donors were analysed for lipids, Lp(a), and apolipoproteins apo B and apo E. Serum and tissue parameters were correlated. In the aortic tissue, higher Lp(a) and apolipoprotein levels were observed in the diseased samples. The total amount of Lp(a) recovered during three different extraction procedures was 5 micrograms/g wet weight in tissue free of plaque and 11.8 micrograms/g wet weight in atherosclerotic tissue. The corresponding values for apo B and apo E were 4.3 and 6.1 micrograms/g wet weight vs. 5.0 and 9.1 micrograms/g wet weight. After density gradient centrifugation of the aortic tissue extracts, it was shown that the major parts of apo(a) and apo B detected in the lesion-free vessel wall were present as Lp(a)-like particles. In the diseased tissue Lp(a) was partly dissociated into LDL-like particles and free apo(a). With this study we confirm that Lp(a) accumulates in the arterial wall, preferentially in diseased tissue, and that Lp(a) particles, deposited in atherosclerotic plaques, are partly degraded to LDL-like particles and free apo(a) in atherosclerotic plaques.
http://www.jlr.org/cgi/reprint/32/2/317
Journal of Lipid Research Volume 32, 1991 317
Quantification of apo[a] and apoB in human atherosclerotic lesions by Judith M. Pepin et al.
ndation, Cleveland, OH 44195
Lysine Binding Site in Lipoprotein (a) – LBS knockout study
http://www.pubmedcentral.nih.gov/articlerender.fcgi?rendertype=abstract&artid=508329
J Clin Invest. 1997 September 15; 100(6): 1493–1500.
Lipoprotein(a) vascular accumulation in mice. In vivo analysis of the role of lysine binding sites using recombinant adenovirus by S D Hughes et al.
Although the mechanism by which lipoprotein(a) [Lp(a)] contributes to vascular disease remains unclear, consequences of its binding to the vessel surface are commonly cited in postulated atherogenic pathways. Because of the presence of plasminogen-like lysine binding sites (LBS) in apo(a), fibrin binding has been proposed to play an important role in Lp(a)’s vascular accumulation. Indeed, LBS are known to facilitate Lp(a) fibrin binding in vitro. To examine the importance of apo(a) LBS in Lp(a) vascular accumulation in vivo, we generated three different apo(a) cDNAs: (a) mini apo(a), based on wild-type human apo(a); (b) mini apo(a) containing a naturally occurring LBS defect associated with a point mutation in kringle 4-10; and (c) human- rhesus monkey chimeric mini apo(a), which contains the same LBS defect in the context of several additional changes. Recombinant adenovirus vectors were constructed with the various apo(a) cDNAs and injected into human apoB transgenic mice. At the viral dosage used in these experiments, all three forms of apo(a) were found exclusively within the lipoprotein fractions, and peak Lp(a) plasma levels were nearly identical (approximately 45 mg/dl). In vitro analysis of Lp(a) isolated from the various groups of mice confirmed that putative LBS defective apo(a) yielded Lp(a) unable to bind lysine-Sepharose. Quantitation of in vivo Lp(a) vascular accumulation in mice treated with the various adenovirus vectors revealed significantly less accumulation of both types of LBS defective Lp(a), relative to wild-type Lp(a). These results indicate a correlation between lysine binding properties of Lp(a) and vascular accumulation, supporting the postulated role of apo(a) LBS in this potentially atherogenic characteristic of Lp(a).
Collagen, Lysine, Lipoprotein (a)
http://www-personal.umich.edu/~egatenby/collagen%20eyre%20chapter.pdf
collagen and lysine crosslinking images
Basement membrane collagens are ancient (>500 million years [50, 51]), having evolved in primitive metazoa as early or earlier than the fibril-forming collagens. Hydra, a simple organism formed from two cell layers that secrete and sandwich the mesoglea, an extracellular layer, has been shown to express genes for a basement membrane collagen that is homologous in sequence to vertebrate type IV collagen and for a fibril-forming collagen.
Collagen lysyl hydroxylase is an ascorbate-dependent enzyme that hydroxylates lysyl residues on collagen neopeptides.
Role of Vitamin C in Hydroxylation of Lysine and Proline Crosslinking in Collagen Fibers
http://en.wikipedia.org/wiki/Ascorbate
Ascorbate WIkipedia, Function In humans,
vitamin C is a highly effective antioxidant, acting to lessen oxidative stress; a substrate for ascorbate peroxidase[4]; and an enzyme cofactor for the biosynthesis of many important biochemicals.
Vitamin C acts as an electron donor for eight different enzymes:[9]
Three participate in collagen hydroxylation.[10][11][12] These reactions add hydroxyl groups to the amino acids proline or lysine in the collagen molecule (via prolyl hydroxylase and lysyl hydroxylase), thereby allowing the collagen molecule to assume its triple helix structure and making vitamin C essential to the development and maintenance of scar tissue, blood vessels, and cartilage.[13]
Two are necessary for synthesis of carnitine.[14][15] Carnitine is essential for the transport of fatty acids into mitochondria for ATP generation.
The remaining three have the following functions: dopamine beta hydroxylase participates in the biosynthesis of norepinephrine from dopamine.[16][17] another enzyme adds amide groups to peptide hormones, greatly increasing their stability.[18][19] one modulates tyrosine metabolism.[20][21]
Biological tissues that accumulate over 100 times the level in blood plasma of vitamin C are the adrenal glands, pituitary, thymus, corpus luteum, and retina.[22] Those with 10 to 50 times the concentration present in blood plasma include the brain, spleen, lung, testicle, lymph nodes, liver, thyroid, small intestinal mucosa, leukocytes, pancreas, kidney and salivary glands.
GLO Gulano Lactone Oxidase Genetic Defect
http://www.ncbi.nlm.nih.gov/pubmed/10572964
Biochim Biophys Acta. 1999 Oct 18;1472(1-2):408-11.
Random nucleotide substitutions in primate nonfunctional gene for L-gulono-gamma-lactone oxidase, the missing enzyme in L-ascorbic acid biosynthesis. Ohta Y, Nishikimi M.
Humans and other primates have no functional gene for L-gulono-gamma-lactone oxidase that catalyzes the last step of L-ascorbic acid biosynthesis. The 164-nucleotide sequence of exon X of the gene was compared among human, chimpanzee, orangutan, and macaque, and it was found that nucleotide substitutions had occurred at random throughout the sequence with a single nucleotide deletion, indicating that the primate L-gulono-gamma-lactone oxidase genes are a typical example of pseudogene.
http://www.jbc.org/cgi/content/abstract/269/18/13685
J. Biol. Chem., Vol. 269, Issue 18, 13685-13688, 05, 1994
Cloning and chromosomal mapping of the human nonfunctional gene for L- gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. by M Nishikimi et al.
Man is among the exceptional higher animals that are unable to synthesize L-ascorbic acid because of their deficiency in L-gulono- gamma-lactone oxidase, the enzyme catalyzing the terminal step in L- ascorbic acid biosynthesis.
Genetically Engineered Vitamin C Deficient Mouse Model shows disruption of Collagen Fibers in Aorta and Carotid Bifurcation.
http://www.pnas.org/content/97/2/841.full
PNAS January 18, 2000 vol. 97 no. 2 841-846
Aortic wall damage in mice unable to synthesize ascorbic acid by Nobuyo Maeda et al.
By inactivating the gene for l-gulono-γ-lactone oxidase, a key enzyme in ascorbic acid synthesis, we have generated mice that, like humans, depend on dietary vitamin C. Regular chow, containing about 110 mg/kg of vitamin C, is unable to support the growth of the mutant mice, which require l-ascorbic acid supplemented in their drinking water (330 mg/liter). Upon withdrawal of supplementation, plasma and tissue ascorbic acid levels decreased to 10–15% of normal within 2 weeks, and after 5 weeks the mutants became anemic, began to lose weight, and die. Plasma total antioxidative capacities were approximately 37% normal in homozygotes after feeding the unsupplemented diet for 3–5 weeks. As plasma ascorbic acid decreased, small, but significant, increases in total cholesterol and decreases in high density lipoprotein cholesterol were observed.
The most striking effects of the marginal dietary vitamin C were alterations in the wall of aorta, evidenced by the disruption of elastic laminae, smooth muscle cell proliferation, and focal endothelial desquamation of the luminal surface. Thus, marginal vitamin C deficiency affects the vascular integrity of mice unable to synthesize ascorbic acid, with potentially profound effects on the pathogenesis of vascular diseases. Breeding the vitamin C-dependent mice with mice carrying defined genetic mutations will provide numerous opportunities for systematic studies of the role of antioxidants in health and disease.
Rupture of Elastic Lamina, Smooth Muscle Cell Proliferation, and Injury of the Luminal Surface in the Thoracic Aorta.
Ascorbic acid is an important cofactor for the hydroxylation of proline and lysine necessary for the crosslinking of collagen and elastin, important structural components of vessel wall (18, 19). Inspection under both light microscopy and TEM of cross sections of the thoracic aorta from animals with low levels of plasma and tissue ascorbic acid revealed marked alterations in the pattern and integrity of the elastic laminae. Prominent breaks and fragmentation of the elastica were located in both the superficial and deep media (Fig. 5 . The endothelial cells overlying the areas of internal elastic lamia disruption were attenuated as a result of the accumulation of basement membrane material, collagen/elastic tissue, and aggregates of smooth muscle cells. Some smooth muscle cells had an altered morphology, were devoid of cytoplasmic filaments, and contained myelin figures indicative of cellular degeneration (not shown). Small clusters of smooth muscle cells, ranging from a few to a diffuse collections of several cells, were present within the subintima below the basement membrane and above the superficial elastic lamina (Fig. 5 C). Most of the smooth muscle cells present within the intima were mildly activated as judged by a slight increase in their rough endoplasmic reticulum. Small collections of elastic fibers were located between smooth muscle cells both in the intima and in the deep media, suggesting that reorganization of the elastic lamina is a dynamic process in these mice.
The most striking pathological finding in our current study is the presence of abnormalities in the aortic walls of the vitamin C-deficient mice. The simplest explanation of this pathology is that the fragmentation of elastic lamina is caused by defects in the crosslinking of collagen and elastin because of the need for vitamin C to generate hydroxylysine and hydroxyproline.
Support for this explanation is provided by the observations of similar fragmentation of elastic fibers in mice with the Blotchy allele at the X-linked Mottled locus (33). The Mottled locus codes for a copper-transporting ATPase, which is necessary for lysyl oxidase activity to crosslink collagen and elastin (34).
Similar aortic wall changes also have been seen in young rats treated with β-aminopropionitrile, an inhibitor of lysyl oxidase (35, 36). However, although the aortic wall abnormalities in Blotchy mice progress to gross dilatation, aneurysm, and aortic rupture, the abnormalities seen in our vitamin C-deficient mice appear to be more subtle.
The lesions in the aortic arch of vitamin C-deficient mice are longitudinal and most frequently are seen near the takeoff of the carotid where the blood flow related shear-stress is high. We did not find platelets, fibrin, or other blood cells in the areas with endothelial alteration. But there is a marked increase in the extravasation of macromolecules into the aortic wall in these areas as evidenced by the distribution of Evans blue in the vitamin C-deficient mice.
In conclusion, we have generated mice lacking l-gulono-γ-lactone oxidase, a key enzyme for ascorbic acid synthesis. The mutant mice, like humans, entirely depend on dietary vitamin C, and they show changes indicating that the integrity of their vasculature is compromised. When combined with the batteries of mutant mice generated in recent years, the Gulo −/− mutant mouse should provide unique opportunities for exploring the interactions between genetic determination and environmental factors, such as oxidative stress, and the effects on these interactions of different levels of vitamin C in the diet.
Collagen and Ascorbate a Review
http://www.ncbi.nlm.nih.gov/pubmed/3008449
Yale J Biol Med. 1985 Nov-Dec;58(6):553-9.Related Articles, Links
Regulation of collagen biosynthesis by ascorbic acid: a review. by Pinnell SR.
L-ascorbic acid is an essential cofactor for lysyl hydroxylase and prolyl hydroxylase, enzymes essential for collagen biosynthesis. In addition, L-ascorbic acid preferentially stimulates collagen synthesis in a manner which appears unrelated to the effect of L-ascorbic acid on hydroxylation reactions. This reaction is stereospecific and unrelated to intracellular degradation of collagen. The effect apparently occurs at a transcriptional or translational level, since L-ascorbic acid preferentially stimulates collagen-specific mRNA. In addition, it stimulates lysyl hydroxylase activity but inhibits prolyl hydroxylase activity in human skin fibroblasts in culture.
Lysine Binding Sites on Liporotein (a) – Fivefold reduction in atherosclerosis when Lysine Binding Sites Eliminated with Mutation.
http://www.jci.org/articles/view/119565
Published in Volume 100, Issue 3 J. Clin. Invest. 100(3): 558-564 (1997).
Modification of apolipoprotein(a) lysine binding site reduces atherosclerosis in transgenic mice. N W Boonmark et al.
Lipoprotein(a) contributes to the development of atherosclerosis through the binding of its plasminogen-like apolipoprotein(a) component to fibrin and other plasminogen substrates. Apolipoprotein(a) contains a major lysine binding site in one of its kringle domains. Destruction of this site by mutagenesis greatly reduces the binding of apolipoprotein(a) to lysine and fibrin. Transgenic mice expressing this mutant form of apolipoprotein(a) as well as mice expressing wild-type apolipoprotein(a) have been created in an inbred mouse strain. The wild-type apolipoprotein(a) transgenic mice have a fivefold increase in the development of lipid lesions, as well as a large increase in the focal deposition of apolipoprotein(a) in the aorta, compared with the lysine binding site mutant strain and to nontransgenic littermates. The results demonstrate the key role of this lysine binding site in the pathogenic activity of apolipoprotein(a) in a murine model system.
http://www.knockoutscience.com/showabstract.php?pmid=9239402
J Clin Invest (1997) 100: 558-64. Modification of apolipoprotein(a) lysine binding site reduces atherosclerosis in transgenic mice by NW Boonmark et al. (additional link to same article)
Collagen Triple Helix
http://www.ohiolink.edu/etd/send-pdf.cgi/Person%20Margaret%20M.pdf?acc_num=ysu1196173663
Collagen consists of three polypeptide chains, termed alpha chains, which are arranged in a parallel triple helix. There are two α1 chains and one α2 chain (Goodsell, 2003). Although each chain has a helical arrangement, they only have this conformation when associated with the two other alpha chains and is then referred to as possessing a superhelical configuration (Goodsell, 2003). This superhelical arrangement, consisting of primarily Collagen I and III fibers form an elaborate network between nearly all cells and constitutes the major structural element in bone, tendon, cartilage and teeth.
http://www.cqs.com/cvd.htm
A Simple Preventive and Therapy for Cardiovascular Disease (CVD) Jonathan Campbell, Health Consultant Natural Therapies for Chronic Illness & Health Maintenance
http://www.lef.org/Vitamins-Supplements/Item02117/L-Proline-L-Lysine.html
L-Proline, L-Lysine 275 mg/275 mg, 120 tablets Item Catalog Number: 2117 $14.16
RETINAL PHOTOS CONFIRM VITAMIN C PILLS CAN REVERSE ARTERY DISEASE
http://www.hullcontactlensclinic.co.uk/cardior.htm
CardioRetinometry Dr. Sydney J Bush. PhD. DOpt. (IOSc. London) Optic nerve heads (Disc) Before Vitamin C 2002, After Vitamin C 2004, Un-retouched images showing retinal arteries opening and veins carrying more blood.. The white line down the arteries is lighter and thinner showing less cholesterol in the 2004 image. which also shows ‘lost’ vessels reappearing. This is what is now said to happen in heart muscle as new anastomoses open when arteries become blocked.
http://www.bmj.com/cgi/eletters/329/7457/79#68348
CardioRetinometry 23 July 2004 Sydney J Bush, Optometrist. CardioRetinometrist 20-22 Brook St. HULL HU2 8LA
Wong TY et al. Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ 2004 329:79 (10th July)pub 2nd Jne.
Bush SJ. Cardioretinometry Rapid Response BMJ 23rd July
Bush SJ. “Emperor’s New Clothes?” Rapid Response 25th Nov. 2004
Bush SJ. ‘Optician’ Letters 9th May 2003 and later in 2004.
Guinea Pig and GLO defect
http://en.wikipedia.org/wiki/Guinea_pig
Like humans, but unlike most other mammals, guinea pigs cannot synthesize their own vitamin C and must obtain this vital nutrient from food. If guinea pigs do not ingest enough vitamin C, they can suffer from potentially fatal scurvy.
http://www.ncbi.nlm.nih.gov/pubmed/14703305
Inai Y et al. (2003), “The Whole Structure of the Human Non-Functional L-Guluno-gamma-Lactone Oxidase Gene – the Gene Responsible for Scurvy – and the Evolution of Repetitive Sequences Thereon,” J Nutr Sci Vitaminology 49:315-319.
The whole structure of the human nonfunctional L-gulono-gamma-lactone oxidase gene–the gene responsible for scurvy–and the evolution of repetitive sequences thereon. Inai Y, Ohta Y, Nishikimi M.
L-Gulono-gamma-lactone oxidase (GULO), which catalyzes the last step of ascorbic acid biosynthesis, is missing in humans. The whole structure of the human gene homologue for this enzyme was disclosed by a computer-assisted search. Only five exons, as compared to 12 exons constituting the functional rat GULO gene, remain in the human genome. A comparison of these exons with those of their functional counterparts in rat showed that there are two single nucleotide deletions, one triple nucleotide deletion, and one single nucleotide insertion in the human sequence. When compared in terms of codons, the human sequence has a deletion of a single amino acid, two stop codons, and two aberrant codons missing one nucleotide besides many amino acid substitutions. A comparison of the remaining human exon sequences with the corresponding sequences of the guinea pig nonfunctional GULO gene revealed that the same substitutions from rats to both species occurred at a large number of nucleotide positions. From analyses of the molecular evolution of Alu sequences in the human GULO gene homologue, it is thought that two Alu sequences were inserted in the vicinity of a presumed position of lost exon 11 during the same period as GULO lost its function. It is predicted that six LINE-1 sequences located in and near the gene homologue were inserted not during that period.
http://www.jbc.org/cgi/content/abstract/267/30/21967
J. Biol. Chem., Vol. 267, Issue 30, 21967-21972, Oct, 1992
Guinea pigs possess a highly mutated gene for L-gulono-gamma-lactone oxidase, the key enzyme for L-ascorbic acid biosynthesis missing in this species
M Nishikimi, T Kawai and K Yagi
Heart Disease in US declined after 1970 publication of Linus Pauling Book on Vitamin C, Vitamin C and the Common Cold.
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm4830a1.htm
MMWR Weekly – Decline in CVD Death Rates, Achievements in Public Health, 1900-1999: Decline in Deaths from Heart Disease and Stroke — United States, 1900-1999
http://www.vitamincfoundation.org/forum/index.php
Vitmain C Foundation Message Board
Link to this article: http://wp.me/P3gFbV-qL
http://jeffreydach.com/2008/11/27/heart-disease-ascorbate-lysine-and-linus-pauling-by-jeffrey-dach-md.aspx
Jeffrey Dach MD
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.truemedmd.com
Disclaimer
http://www.drdach.com/wst_page20.html www.drdach.com disclaimer
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur .
(c) 2008-2013 Jeffrey Dach MD All Rights Reserved This article may be reproduced on the internet without permiss
Familial Hypercholesterolemia and Statin Drugs by Jeffrey Dach MD - Jeffrey Dach MD July 5, 2013 at 11:39 AM
[…] Heart Disease Vitamin C and Linus Pauling […]