 Anti-Cancer Activity from Natural Plant Pterostilbenes
Anti-Cancer Activity from Natural Plant Pterostilbenes
by Jeffrey Dach MD
This article is
Part Three of a Series.
For Part One Click Here.
For Part Two Click Here.
Epidemiological studies showing a diet high in fruits and vegetables reduced cancer risk sparked interest in commonly available foods as anti-cancer agents.
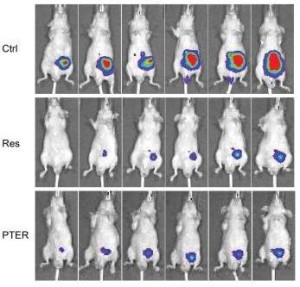
Above left image: Inhibition of cancer growth. Male Mice were injected with Prostate Cancer cells, and then treated with placebo (CTRL – controls upper row), Resveratrol (RES – middle row) or Pterostilbene (PTER lower row) . Color intensity shows cancer inhibition by Res and PTER. (5)
In 1997, Jang reported in Science the anti-cancer effects of Resveratrol, present in grapes and berries, an anti-cancer, anti-inflammatory, and anti-aging food supplement.
Resveratrol Analogs
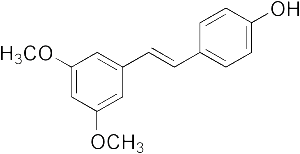
In recent years, a number of analogs of Resveratrol called stilbenes have been recognized as more suitable as anti-cancer agents. In particlular, Piceatannol a hydroxylated version of Resveratrol, and Pterostilbene a methoxylated version of Resveratrol have been the focus of a flurry of research showing in-vitro and in- vivo anti-cancer activity. Pterostilbene is available at the vitamin store as a food supplement. This article will explain and summarize some of these studies.

Left Image : Pterostilbene , available as a Nutritional Supplement, Photo courtesy of Source Naturals.
The USDA
The USDA and the University of Mississippi have been studying Resveratrol analogs for more than a decade, and in 2002, reported inhibition of breast cancer in a mouse model (1). This same group delved into the molecular biology of Pterostilbene’s anti-cancer activity and came out with two important papers this year (in 2013) (4,5).
In the first paper, the authors examined the anti-cancer activity of various analogs of Resveratrol (Piceatannol, and 3M-Resveratrol) in prostate cancer cells, both of which showed higher potency in inhibiting tumor progression compared to Resveratrol itself. They concluded that their “findings offer strong pre-clinical evidence for the utilization of dietary stilbenes, particularly 3M-Res, as novel, potent, effective chemopreventive agents in prostate cancer“.(4)
Pterostilbene IS The Most Promising
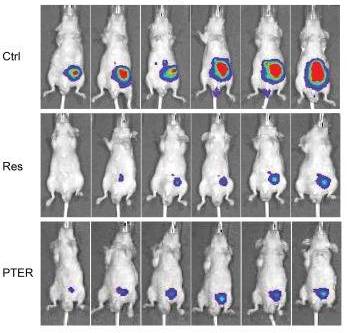
In their next study, Dr Li reports that Pterostilbene appears to be the most promising of the Resveratrol analogs, which significantly inhibited tumor growth, progression, local invasion and spontaneous metastasis in a mouse model of prostate cancer. (5)
 Additional studies have been done on other cancers such as Lung Cancer (7,10) Breast Cancer (8,9,11,16) Colon Cancer (13) skin cancer (20) and Leukemia (17).
Additional studies have been done on other cancers such as Lung Cancer (7,10) Breast Cancer (8,9,11,16) Colon Cancer (13) skin cancer (20) and Leukemia (17).
Left Image is chemical structure of Pterostilbene courtesy of wikimedia commons
Mechanism of action:
Studies show that Pterostilbene induces apoptosis via the mitochondrial pathway in breast cancer cell lines. See the article by Moon. Another article by Pei-Ching Hsiao. His study used acute myeloid leukemia cells, finding pterostilbene induced apoptosis in cancer cells vIa mitochondrial pathways, (with activation of caspase system). Another study by Alosi on Pterostilbene in Breast Cancer also showed similar findings with apoptosis induce by mitochondrial pathways.
Another more recent study by Wang in 2012 showed Pterostilbene induces apoptosis and cell cycle arrest in breast cancer cells.
Conclusion: Pterostilbene is a compound found in grapes and berries which have striking anti-cancer activity in animal models through mechanisms elucidated by modern molecular biology. These are not drugs. Rather, they are safe food supplements available at the vitamin store without a prescription. Other health benefits such as blood sugar control, lipid control and blood pressure modulation will be the topic for part two of this series.
This article is Part Three of a Series.
For Part One Click Here.
For Part Two Click Here.
Articles with related interest:
Iodine for Breast Cancer Prevention and Treatment
Links and References
J Agric Food Chem. 2002 Jun 5;50(12):3453-7. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. Rimando AM, Cuendet M, Desmarchelier C, Mehta RG, Pezzuto JM, Duke SO. Natural Products Utilization Research Unit, Agricultural Research Service, U.S. Department of Agriculture, P.O. Box 8048, University, Mississippi 38677, USA.
Pterostilbene, a natural methoxylated analogue of resveratrol, was evaluated for antioxidative potential. The peroxyl-radical scavenging activity of pterostilbene was the same as that of resveratrol, having total reactive antioxidant potentials of 237 +/- 58 and 253 +/- 53 microM, respectively. Both compounds were found to be more effective than Trolox as free radical scavengers. Using a plant system, pterostilbene also was shown to be as effective as resveratrol in inhibiting electrolyte leakage caused by herbicide-induced oxidative damage, and both compounds had the same activity as alpha-tocopherol. Pterostilbene showed moderate inhibition (IC50 = 19.8 microM) of cyclooxygenase (COX)-1, and was weakly active (IC50 = 83.9 microM) against COX-2, whereas resveratrol strongly inhibited both isoforms of the enzyme with IC50 values of approximately 1 microM. Using a mouse mammary organ culture model, carcinogen-induced preneoplastic lesions were, similarly to resveratrol, significantly inhibited by pterostilbene (ED50 = 4.8 microM), suggesting antioxidant activity plays an important role in this process.
2010
2) Pterostilbene_Monograph_Altern_Med_Review_July_2010
Altern Med Rev. 2010 Jul;15(2):159-63.
Pterostilbene. Monograph.[No authors listed] – Excellent Review Article
2012
3) http://www.ncbi.nlm.nih.gov/
J Surg Res. 2012 Apr;173(2):e53-61. doi: 10.1016/j.jss.2011.09.054. Epub 2011 Oct 21.
Pterostilbene and cancer: current review. McCormack D, McFadden D.Department of Surgery, Danbury Hospital, Danbury, Connecticut 06810, USA.
Pterostilbene (trans-3,5-dimethoxy-4-
2013
They examined the anti-proliferative activities of Res/analogues in three PCa cell lines
Dias, Steven J., et al. “Trimethoxy‐Resveratrol and Piceatannol Administered Orally Suppress and Inhibit Tumor Formation and Growth in Prostate Cancer Xenografts.” The Prostate (2013).U.S. Department of Agriculture, University of Mississippi
METHODS We synthesized several natural and synthetic analogues of Res and characterized their effects on PCa cells in vitro using a cell proliferation assay. A colony formation assay and in vitro validation of luciferase (Luc) activity was done for LNCaP-Luc cells that were consequently used for in vivo studies. The efficacy of Res, trimethoxy-resveratrol (3M-Res) and piceatannol (PIC) was studied in a subcutaneous (s.c.) model of PCa using oral gavage. Tumor progression was monitored by traditional caliper and bioluminescent imaging. The levels of cytokines in serum were examined by ELISA, and the levels of compounds in serum and tumor tissues were determined by gas chromatography-mass spectrometry.
RESULTS We examined the anti-proliferative activities of Res/analogues in three PCa cell lines. We further compared the chemopreventive effects of oral Res, 3M-Res, and PIC in LNCaP-Luc-xenografts. We found that 2 weeks pretreatment with the compounds diminished cell colonization, reduced tumor volume, and decreased tumor growth in the xenografts. Both 3M-Res and PIC demonstrated higher potency in inhibiting tumor progression compared to Res. Notably, 3M-Res was the most active in inhibiting cell proliferation and suppressing colony formation, and its accumulation in both serum and tumor tissues was the highest.
CONCLUSIONS Our findings offer strong pre-clinical evidence for the utilization of dietary stilbenes, particularly 3M-Res, as novel, potent, effective chemopreventive agents in PCa. Prostate
Li, K., Dias, S. J., Rimando, A. M., Dhar, S., Mizuno, C. S., Penman, A. D., … & Levenson, A. S. (2013). Pterostilbene Acts through Metastasis-Associated Protein 1 to Inhibit Tumor Growth, Progression and Metastasis in Prostate Cancer.
Am J Surg. 2013 Apr;205(4):483.Pterostilbene and its emerging antineoplastic effects: a prospective treatment option for systemic malignancies. Kapoor S.
7) http://www.bioportfolio.com/
Chemopreventive Effects of Pterostilbene on Urethane-Induced Lung Carcinogenesis in Mice via the Inhibition of EGFR-Mediated Pathways and the Induction of Apoptosis and Autophagy. Department of Environmental and Occupational Health, National Cheng Kung University Medical College , Tainan, Taiwan.
Journal of agricultural and food chemistry
The aim of this study is to investigate the chemopreventive effects of pterostilbene in urethane-induced murine lung tumors. Pretreatment with pterostilbene at 50 or 250 mg/kg significantly reduced tumor multiplicity by 26 and 49%, respectively. Pterostilbene also significantly inhibited tumor volume by 25 and 34% and decreased the tumor burden per mouse by 45 and 63%, respectively.
2012
8) http://www.ncbi.nlm.nih.gov/
http://www.ncbi.nlm.nih.gov/
Am J Transl Res. 2012;4(1):44-51. Epub 2012 Jan 5.
Pterostilbene simultaneously induces apoptosis, cell cycle arrest and cyto-protective autophagy in breast cancer cells.Wang Y, Ding L, Wang X, Zhang J, Han W, Feng L, Sun J, Jin H, Wang XJ.
As a nature phytoalexin found in grapes, resveratrol has been proposed as a potential drug for cancer chemoprevention and treatment. However, its poor bioavailability limits its potential clinical application. Pterostilbene, the natural dimethylated analog of resveratrol with greater bioavailability, was confirmed to inhibit tumor growth both in vivo and in vitro, demonstrating its potential for further clinical application. In the current study, we found that pterostilbene could markedly inhibit the growth of two independent breast cancer cell lines. Both apoptosis and cell cycle arrest as well as the inhibition of wnt singling was induced by pterostilbene. The dominant-active mutant of ß-catenin could reverse the growth inhibitory effect of pterostilbene, indicating that the inhibition of wnt signaling is important to the growth inhibitory effect of pterostilbene. Interestingly, pterostilbene induced autophagy and blockage of autophagy augmented pterostilbene-induced growth inhibition, suggesting that the combination of autophagy inhibitors with pterostilbene and other therapeutics such as endocrine drugs could serve as a new and promising strategy for the treatment of breast cancer cells.
2012
9) http://www.ncbi.nlm.nih.gov/
http://www.ncbi.nlm.nih.gov/
PLoS One. 2012;7(9):
Pterostilbene-induced tumor cytotoxicity: a lysosomal membrane permeabilization-dependent mechanism. Mena S, Rodríguez ML, Ponsoda X, Estrela JM, Jäättela M, Ortega AL. Source Green Molecular, Valencia, Spain.
The phenolic phytoalexin resveratrol is well known for its health-promoting and anticancer properties. Its potential benefits are, however, limited due to its low bioavailability. Pterostilbene, a natural dimethoxylated analog of resveratrol, presents higher anticancer activity than resveratrol. The mechanisms by which this polyphenol acts against cancer cells are, however, unclear. Here, we show that pterostilbene effectively inhibits cancer cell growth and stimulates apoptosis and autophagosome accumulation in cancer cells of various origins. However, these mechanisms are not determinant in cell demise.
Pterostilbene promotes cancer cell death via a mechanism involving lysosomal membrane permeabilization. Different grades of susceptibility were observed among the different cancer cells depending on their lysosomal heat shock protein 70 (HSP70) content, a known stabilizer of lysosomal membranes. A375 melanoma and A549 lung cancer cells with low levels of HSP70 showed high susceptibility to pterostilbene, whereas HT29 colon and MCF7 breast cancer cells with higher levels of HSP70 were more resistant. Inhibition of HSP70 expression increased susceptibility of HT29 colon and MCF7 breast cancer cells to pterostilbene. Our data indicate that lysosomal membrane permeabilization is the main cell death pathway triggered by pterostilbene.
2013
10) http://www.ncbi.nlm.nih.gov/
http://www.ncbi.nlm.nih.gov/
PLoS One. 2013 May 3;8(5)
Pterostilbene Exerts Antitumor Activity via the Notch1 Signaling Pathway in Human Lung Adenocarcinoma Cells. Yang Y, Yan X, Duan W, Yan J, Yi W, Liang Z, Wang N, Li Y, Chen W, Yu S, Jin Z, Yi D. Department of Cardiovascular Surgery, Xijing Hospital, The Fourth Military Medical University, Xi’an City, China.
In this study, we investigated the antitumor activity of PTE against human lung adenocarcinoma in vitro and in vivo and explored the role of the Notch1 signaling pathway in this process. PTE treatment resulted in a dose- and time-dependent decrease in the viability of A549 cells. Additionally, PTE exhibited strong antitumor activity, as evidenced not only by a reduced mitochondrial membrane potential (MMP) and a decreased intracellular glutathione content but also by increases in the apoptotic index and the level of reactive oxygen species (ROS). Furthermore, PTE treatment induced the activation of the Notch1 Intracellular Domain (NICD) protein and activated Hes1. DAPT (a gamma secretase inhibitor) and Notch1 siRNA prevented the induction of NICD and Hes1 activation by PTE treatment and sensitized the cells to PTE treatment. The down-regulation of Notch signaling also prevented the activation of pro-survival pathways (most notably the PI3K/Akt pathway) after PTE treatment. In summary, lung adenocarcinoma cells may enhance Notch1 activation as a protective mechanism in response to PTE treatment.
2010
11) http://www.ncbi.nlm.nih.gov/
J Surg Res. 2010 Jun 15;161(2):195-201.
Pterostilbene inhibits breast cancer in vitro through mitochondrial depolarization and induction of caspase-dependent apoptosis. Alosi JA, McDonald DE, Schneider JS, Privette AR, McFadden DW. University of Vermont, Burlington, Vermont, USA.
Epidemiologic studies suggest that diets high in fruits and vegetables reduce cancer risk. Resveratrol, a compound present in grapes, has been shown to inhibit a variety of primary tumors. Pterostilbene, an analogue of resveratrol found in blueberries, has both antioxidant and antiproliferative properties. We hypothesized that pterostilbene would induce apoptosis and inhibit breast cancer cell growth in vitro.
METHODS: Breast cancer cells were treated with graduated doses of pterostilbene. Cell viability was measured by MTT assay. Apoptosis was evaluated via DNA fragmentation assay and TUNEL assay. Apo-ONE caspase-3/7 assay was used to evaluate caspase activity. Flow cytometry was used to evaluate mitochondrial depolarization, superoxide formation, and cell cycle. Student’s t-test and two-way ANOVA with Bonferroni posttests were utilized for statistical analysis.
RESULTS: Pterostilbene decreased breast cancer cell viability in a concentration- and time-dependent manner. Pterostilbene treatment increased caspase-3/7 activity and apoptosis in both cell lines. Caspase-3/7 inhibitors completely reversed pterostilbene’s effects on cell viability. Pterostilbene treatment triggered mitochondrial depolarization, increased superoxide anion, and caused alteration in cell cycle.
CONCLUSIONS: Pterostilbene treatment inhibits the growth of breast cancer in vitro through caspase-dependent apoptosis. Mitochondrial membrane
depolarization and increased superoxide anion may contribute to the activation downstream effector caspases. Caspase inhibition leads to complete reversal of pterostilbene’s effect on cell viability. Further in vitro mechanistic studies and in vivo experiments are warranted to determine its potential for the treatment of breast cancer.
2006
12) Pharmacometrics_of_Pterostilbenes_Curr_Clin_Pharmacol_2006_Davies
Curr Clin Pharmacol. 2006 Jan;1(1):81-101.
Pharmacometrics of stilbenes: seguing towards the clinic.
Roupe KA, Remsberg CM, Yáñez JA, Davies NM.
Department of Pharmaceutical Sciences, College of Pharmacy, Washington State University, Pullman, Washington 99164-6534, USA.
Stilbenes are small molecular weight (approximately 200-300 g/mol), naturally occurring compounds and are found in a wide range of plant sources, aromatherapy products, and dietary supplements. These molecules are synthesized via the phenylpropanoid pathway and share some structural similarities to estrogen. Upon environmental threat, the plant host activates the phenylpropanoid pathway and stilbene structures are produced and subsequently secreted. Stilbenes act as natural protective agents to defend the plant against viral and microbial attack, excessive ultraviolet exposure, and disease. One stilbene, resveratrol, has been extensively studied and has been shown to possess potent anti-cancer, antiinflammatory and anti-oxidant activities. Found primarily in the skins of grapes, resveratrol is synthesized by Vitis vinifera grapevines in response to fungal infection or other environmental stressors. Considerable research showing resveratrol to be an attractive candidate in combating a wide variety of cancers and diseases has fueled interest in determining the disease-fighting capabilities of other structurally similar stilbene compounds. The purpose of this review is to describe four such structurally similar stilbene compounds, piceatannol, pinosylvin, rhapontigenin, and pterostilbene and detail some current pharmaceutical research and highlight their potential clinical applications.
13) Pterostilbene_suppresses_aberrant_crypt_colon_carcinogenesis
Clin Cancer Res. 2007 Jan 1;13(1):350-5.
Pterostilbene, an active constituent of blueberries, suppresses aberrant crypt foci formation in the azoxymethane-induced colon carcinogenesis model in rats.
Suh N, Paul S, Hao X, Simi B, Xiao H, Rimando AM, Reddy BS.
Source Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, New Jersey 08854, USA.
Epidemiologic studies have linked the consumption of fruits and vegetables to reduced risk of several types of cancer. Laboratory animal model studies have provided evidence that stilbenes, phenolic compounds present in grapes and blueberries, play a role in inhibiting the risk of certain cancers. Pterostilbene, a naturally occurring stilbene from blueberries, was tested for its preventive activity against colon carcinogenesis.
EXPERIMENTAL DESIGN: Experiments were designed to study the inhibitory effect of pterostilbene against the formation of azoxymethane-induced colonic aberrant crypt foci (ACF) preneoplastic lesions in male F344 rats. Beginning at 7 weeks of age, rats were treated with azoxymethane (15 mg/kg body weight s.c., once weekly for 2 weeks). One day after the second azoxymethane treatment, rats were fed experimental diets containing 0 or 40 ppm of pterostilbene. At 8 weeks after the second azoxymethane treatment, all rats were sacrificed, and colons were evaluated for ACF formation and for inhibition of inducible nitric oxide synthase (iNOS) and proliferating cell nuclear antigen. Effects on mucin MUC2 were also determined.
RESULTS: Administration of pterostilbene for 8 weeks significantly suppressed azoxymethane-induced formation of ACF (57% inhibition, P < 0.001) and multiple clusters of aberrant crypts (29% inhibition, P < 0.01). Importantly, dietary pterostilbene also suppressed azoxymethane-induced colonic cell proliferation and iNOS expression. Inhibition of iNOS expression by pterostilbene was confirmed in cultured human colon cancer cells.
CONCLUSIONS: The results of the present study suggest that pterostilbene, a compound present in blueberries, is of great interest for the prevention of colon cancer.
14) Pharmacometrics_of_pterostilbene_Phytother_Res_2008
Phytother Res. 2008 Feb;22(2):169-79.
Pharmacometrics of pterostilbene: preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Remsberg CM, Yáñez JA, Ohgami Y, Vega-Villa KR, Rimando AM, Davies NM. Department of Pharmaceutical Sciences, College of Pharmacy, Washington State University Pullman, Washington 99164-6534, USA.
The present study evaluated the preclinical pharmacokinetics and pharmacodynamics of trans-pterostilbene, a constituent of some plants. Right jugular vein cannulated male Sprague-Dawley rats were dosed i.v. with 20 mg/kg of pterostilbene and samples were analysed by the reverse phase HPLC method. Serum AUC, serum t(1/2), urine t(1/2), Cl(total) and Vd(beta) were 17.5 +/- 6.6 microg/h/mL, 1.73 +/- 0.78 h, 17.3 +/- 5.6 h, 0.960 +/- 0.025 L/h/kg and 2.41 +/- 1.13 L/kg (mean +/- SEM), respectively. A pterostilbene glucuronidated metabolite was detected in both serum and urine. The in vitro metabolism in rat liver microsomes furthermore suggests phase II metabolism of pterostilbene. Pterostilbene demonstrated concentration-dependent anticancer activity in five cancer cell lines (1-100 microg/mL). An in vitro colitis model showed concentration-dependent suppression of PGE(2) production in the media of HT-29 cells. Antiinflammatory activity was examined by inducing inflammation in canine chondrocytes followed by treatment with pterostilbene (1-100 microg/mL). The results showed decreased levels of MMP-3, sGAG and TNF-alpha compared with control levels. Pterostilbene exhibited concentration-dependent antioxidant capacity measured by the ABTS method. Pterostilbene increased the latency period to response in both tail-flick and hot-plate analgesic tests.
15) Resveratrol_derivatives_cancer_Drug_Discov_Today_2010_Fulda
Drug Discov Today. 2010 Sep;15(17-18):757-65.
Resveratrol and derivatives for the prevention and treatment of cancer. Fulda S. Institute for Experimental Cancer Research in Pediatrics, Goethe-University, D-60528 Frankfurt, Germany.
There are several natural derivatives of resveratrol that are structurally similar to resveratrol and are also present in food. Such resveratrol derivatives might provide promising tools as cancer chemopreventive agents, as well as cancer therapeutics in the prevention and treatment of cancer. This review provides an overview of key derivatives of resveratrol as cancer therapeutics.
16) http://www.hindawi.com/journals/ecam/2011/562187/
Evidence-Based Complementary and Alternative Medicine Volume 2011 (2011), Suppression of Heregulin-β1/HER2-Modulated Invasive and Aggressive Phenotype of Breast Carcinoma by Pterostilbene via Inhibition of Matrix Metalloproteinase-9, p38 Kinase Cascade and Akt Activation
Min-Hsiung Pan,1 Ying-Ting Lin,2 Chih-Li Lin,3 Chi-Shiang Wei,2 Chi-Tang Ho,4 and Wei-Jen Chen21Department of Seafood Science, National Kaohsiung Marine University, Nan-Tzu, Kaohsiung, Taiwan 2Department of Biomedical Sciences, Chung Shan Medical University, No. 110, Section 1, Chien-Kuo N. Road, Taichung 402, Taiwan 3Institute of Medicine, Chung Shan Medical University, Taichung 402, Taiwan 4Department of Food Science, Cook College, Rutgers University, New Brunswick, NJ, USA
17) Pterostilbene_apoptosis_ leukemia
http://www.ncbi.nlm.nih.gov/pubmed/23264221
Folia Histochem Cytobiol. 2012;50(4):574-80.
Pterostilbene induces cell cycle arrest and apoptosis in MOLT4 human leukemia cells. Siedlecka-Kroplewska K, Jozwik A, Kaszubowska L, Kowalczyk A, Boguslawski W. Department of Histology, Medical University of Gdansk, Gdansk, Poland.
Pterostilbene, a polyphenolic compound present in grapes and other fruits, has been demonstrated to inhibit growth and induce apoptosis and autophagy in some cancer cell types. We found that pterostilbene at the IC(90) concentration of 44 µM inhibited proliferation and induced apoptosis in MOLT4 human leukemia cells. Treatment with pterostilbene resulted in a transient accumulation of cells in the G(0)/G(1)-cell cycle phase followed by the S-phase arrest. Pterostilbene-induced apoptotic death of MOLT4 cells was mediated by caspase-3 activation and was accompanied by the disruption of mitochondrial membrane potential, phosphatidylserine externalization and internucleosomal DNA fragmentation. Our results suggest that pterostilbene could serve as a potential additional chemotherapeutic agent for the treatment of leukemia.
commercial product monograph
18) Natural-Pterostilbene “NATURAL PTEROSTILBENE.” by MAJEED, MUHAMMED.
19) http://clincancerres.aacrjournals.org/content/16/24/5942.long
Clin Cancer Res. 2010 Dec 15;16(24):5942-8.
Resveratrol: challenges in translation to the clinic–a critical discussion.
Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR.Department of Ophthalmology and Visual Sciences, Eye Research Institute, and Carbone Cancer Center, University of Wisconsin, Madison, Wisconsin 53792, USA.
Abstract
Low cancer survival rates and the serious side effects often associated with current chemotherapeutics highlight the need for new and effective nontoxic anticancer agents. Since 1997 when Jang and colleagues first described resveratrol’s ability to inhibit carcinogenesis, it has consistently proven effective at tumor inhibition in diverse human cancer models. This finding has raised the hope that resveratrol would pioneer a novel class of nontoxic chemotherapeutics. As a consequence of initial basic and preclinical studies, resveratrol is now being extensively promoted in the unregulated nutraceutical sector. However, some fundamental aspects of resveratrol’s action need to be understood before it can be developed into a clinically viable anticancer drug. These areas pertain to the key mechanism(s) by which resveratrol potentiates its antitumor effects. Current research suggests that these mechanisms might be through novel pathways, requiring an understanding of cellular uptake, sentinel targets, and in vivo biological networks. The metabolism of resveratrol and its bioavailability also warrant further consideration in light of recent in vitro and in vivo studies. Finally, we need to appreciate the sorts of information about resveratrol that may translate between different disease entities. We present a critical discussion of these issues and suggest important experiments that could pave the way to the successful translation of resveratrol to the clinic.
20) http://www.ncbi.nlm.nih.gov/pubmed/22842666
Food Funct. 2012 Nov;3(11):1185-94. Pterostilbene, a natural analogue of resveratrol, potently inhibits 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin carcinogenesis. Tsai ML, Lai CS, Chang YH, Chen WJ, Ho CT, Pan MH.Department of Seafood Science, National Kaohsiung Marine University, Nan-Tzu, Kaohsiung 811, Taiwan.
Abstract
We reported previously that pterostilbene, a natural analogue of resveratrol from blueberries, strongly suppressed lipopolysaccharide-induced up-expression of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) in murine macrophages. In this study, we further investigated pterostilbene’s molecular mechanism of action and its anti-tumor properties. Pretreatment with pterostilbene has resulted in the reduction of 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced nuclear translocation of the nuclear factor-κB (NFκB) subunits. Pterostilbene also reduced TPA-induced phosphorylation of IκBα and p65 and caused subsequent degradation of IκBα. Moreover, pterostilbene markedly suppressed TPA-induced activation of extracellular signal-regulated kinase (ERK)1/2, p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK)1/2, phosphatidylinositol 3-kinase (PI3K) and Akt, which are upstream of NFκB and activator protein 1 (AP-1). Furthermore, pterostilbene significantly inhibited 7,12-dimethylbenz[a]anthracene (DMBA)/TPA-induced skin tumor formation measured by the tumor multiplicity of papillomas at 20 weeks. The presented data has, for the first time, revealed that pterostilbene is an effective anti-tumor agent that functions by downregulating inflammatory iNOS and COX-2 gene expression in mouse skin. It is suggested that pterostilbene is a novel functional agent capable of preventing inflammation-associated tumorigenesis.
More references:
Pan MH, Chiou YS, Chen WJ, Wang JM, Badmaev V, Ho CT. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis. 2009;30:1234–1242. [PubMed]
Chiou YS, Tsai ML, Wang YJ, Cheng AC, Lai WM, Badmaev V, Ho CT, Pan MH. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxyme-thane-treated mice. J Agric Food Chem. 2010;58:8833–8841. [PubMed]
Schneider JG, Alosi JA, McDonald DE, McFadden DW. Pterostilbene inhibits lung cancer through induction of apoptosis. J Surg Res. 2010;161:18–22. [PubMed]
Alosi JA, McDonald DE, Schneider JS, Privette AR, McFadden DW. Pterostilbene inhibits breast cancer in vitro through mitochondrial depolarization and induction of caspase-dependent apoptosis. J Surg Res. 2010;161:195–201. [PubMed]
Mannal PW, Alosi JA, Schneider JG, McDonald DE, McFadden DW. Pterostilbene inhibits pancreatic cancer in vitro. J Gastrointest Surg. 2010;14:873–879. [PubMed]
Pan MH, Chang YH, Badmaev V, Nagabhushanam K, Ho CT. Pterostilbene induces apoptosis and cell cycle arrest in human gastric carcinoma cells. J Agric Food Chem. 2007;55:7777–7785. [PubMed]
This article is Part Three of a Series.
For Part One Click Here.
For Part Two Click Here.

Jeffrey Dach MD
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Sites and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244124.html
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
disc.yourwebapps.com/Indices/244161.html
disc.yourwebapps.com/Indices/244163.html
disc.yourwebapps.com/Indices/244163.html
health-forums.1
health-forums.2
Disclaimer click here: www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article:http://wp.me/p3gFbV-m3
Copyright (c) 2013 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.









Michael Cleary June 29, 2014 at 2:21 PM
My Wife and I have been eating Salvestrol Platinum 2000 for over ten years. Question, What is the bioavailability of salvestrol. We have been advised by De Montfort University that Resveratrol is merely digested and fails to activate the CYP1B1 protein/enzyme so is a waste of time and money.
I haven’t been able to elucidate the answer either about pterostilbene.
We are told that salvestrol from blueberry, blackberry, red grape and bitter orange is suitable becuse of its excellent bioavailabilty. I am not yet convinced so what is the answer please.
PS We are healthy and as far a we know free of carcinogenisis.
Thank you for your close attention to this lack of knowledge about bioavailability.
Cheers and Kind regards to all of your team.
Michael Cleary (chartered civil and science teacher)