 PCOS The Hidden Epidemic Part Two by Jeffrey Dach MD
PCOS The Hidden Epidemic Part Two by Jeffrey Dach MDThis article is Part Two.of a series.
Click here for Part One of this article.
Click here for Part Three of this article.
Click here for Part Four of this article.
Above left image: Many women with PCOS are frustrated by mainstream medical treatment, and may feel like this photo of Janet Leigh from the Alfred Hitchcock movie, Psycho, (c) copyright 1960 Paramount Pictures.
Cindy, a 34 year old flight attendant had been taking birth control pills for 17 years because of PCOS (Polycystic Ovary Syndrome). The chief complaint was acne and hirsutism from high testosterone levels, for which the birth control pills had been prescribed. Cindy had married a year ago, and was now ready to start a family, so she had discontinued the birth control pills on her own a few months before arriving to my office. Cindy had one normal menstrual period in the last four months since stopping the BCP’s. Cindy came for an office visit because she wanted a treatment that would restore normal menstrual cycles, fertility, as well eliminate the excess facial hair, acne and other androgenic effects of elevated testosterone levels.
Laboratory workup showed absent progesterone production on Day 19 of her cycle indicating anovulation. Genetic testing for CYP21A was negative for nonclassical congenital adrenal hyperplasia. Her DHEA and testosterone levels were mildly elevated. Baseline 17 hydroxyprogesterone was normal. Treatment was started (see below). Three months later Cindy called to report that she was pregnant.
1) Treatment was started with progesterone capsules 100 mg twice a day for days 12-26 of the cycle.
2) Metformin 500 mg tabs twice a day with meals.
3) Clomid 50 mg tabs for 5 days on days 3-7 of the cycle.
4) If the above is ineffective at inducing ovulation, Dexamethazone 2 mg per day for days 3-12 of the cycle.
The defining hallmark of PCOS is lack of ovulation, which in turn causes progesterone deficiency. Progesterone for PCOS was pioneered by John R Lee MD who wrote extensively about his experience treating PCOS with cyclic progesterone. This is covered in Part One of this series. Cyclic progesterone is thought to reset the hypothalamic-pituitary axis which controls ovulation, thus helping restart normal ovulation in the PCOS patient when taken over a 3-6 months of use.
Progesterone inhibits 5 alpha reductase (the enzyme that converts testosterone to its stronger metabolite, DHT), and thus may reduce hirsutism and serve as an anti-androgen. (1)
Spironolactone, (Aldactone 50 mg BID) another commonly used drug used as a blood pressure pill, diuretic pill, and also has anti-androgen effects, it inhibits 5 alpha reductase conversion of testosterone to DHT, and is commonly used to treat hirsutism in PCOS patients. (2)
Metformin is an extremely helpful drug for PCOS patients, and should be considered as the first line of treatment. In obese women with the polycystic ovary syndrome, decreasing serum insulin concentrations with metformin reduces ovarian cytochrome P450c17 alpha activity and ameliorates hyperandrogenism.(3)(13)
Metformin may decrease B12 levels, so make sure you take extra B12 supplements with your Metformin.
Some cases of anovulation are the result of a genetic enzyme deficiency in the adrenal gland with inadequate conversion of 17 hydroxyprogesterone into cortisol. This leads to overproduction of androgenic hormones such as DHEA and Testosterone. This is the CYP 21A genetic mutation, also called the 21 hydroxylase deficiency, also called Non-Classical CAH (congential adrenal hyperplasia) .
This genetic abnormality may be associated with anovulation and hirsutism in a clinical presentation which may mimic PCOS. In this scenario, Dexamethasone may be added to the Clomid. (Dexamethasone 2 mg per day for days 3-12 of the cycle.)
The Dexamethasone inhibits ACTH production by the pituitary and prevents the adrenal from making excess testosterone, and is in fact curative of the hirsutism and acne symptoms, and in many cases restores ovulation and fertility.(6-11)(14-20)
Dexamethasone for Hirsutism and Acne- Relief of Androgenic Symptoms
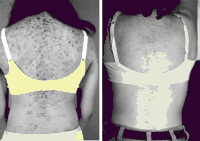 Some authors report success with low dose Dexamethasone (0.25 mg tab PO Qhs) given every night before sleep. This was originally described in an article by Maria New MD in treatment of hirsutism and acne in NON-Classical CAH (congential adrenal hyperplasia) (Maria New MD, JCEM Nov 1, 2006 vol. 91 no. 11 4205-4214)
Some authors report success with low dose Dexamethasone (0.25 mg tab PO Qhs) given every night before sleep. This was originally described in an article by Maria New MD in treatment of hirsutism and acne in NON-Classical CAH (congential adrenal hyperplasia) (Maria New MD, JCEM Nov 1, 2006 vol. 91 no. 11 4205-4214)
(6-11)(14-20)(21)
Above Image: Before and After. Acne Resolves after 3 months of Dexamethasone (0.25 mg Qhs) courtesy of Maria New, JCEM Nov 2006 (21)
Clomiphene is the first drug of choice to induce ovulation in the PCOS patient. Clomid blocks the estrogen receptors in the hypothalamus, causing an LH, FSH surge, which induces ovulation. Clomid is usually given over 5 days during the luteal phase of the cycle, for days 3-7 or 4-8 at a dose starting at 50 mg per day. This may be increased by 100 mg if the lower dosage is unsuccessful and fails to induce ovulation. (4,5) (12,13)
OCPs are commonly prescribed to the PCOS patient to mask the androgenic symptoms of acne and hirsutism. This form of therapy is ill advised and misplaced, since it does not address the underlying problem of lack of ovulation with disordered hormone production. In other words, OCPs are symptomatic treatment which does not address the root cause of the problem, which is anovulation with imbalance in ovarian and adrenal hormone regulation. OCP’s further suppress and prevent ovulation, rather than restore this normal physiological function in the female.
Iodine Supplementation with Lugol’s or Iodoral for the PCOS patient.
Dr. Guy Abraham reports that 12.5-50mg per day of Iodine in the form of Lugol’s solution or Iodoral tablets is beneficial for the PCOS patient, and in many cases will normalize menstrual cycles and restore fertility. He quotes the work of Christine F Skibola who found Iodine from Seaweed beneficial for three pre-menopausal women with menstrual irregularities.
This article is Part Two.of a series.
Click here for Part One of this article.
Click here for Part Three of this article.
Click here for Part Four of this article.
Jeffrey Dach MD
7450 Griffin Road
Suite 190
Davie, Florida 33314
954-792-4663
Links and References
(1) http://www.ncbi.nlm.nih.gov/pubmed/1828548
Obstet Gynecol. 1991 Jul;78(1):103-7.
Effects of sex steroids on skin 5 alpha-reductase activity in vitro.
Cassidenti DL, Paulson RJ, Serafini P, Stanczyk FZ, Lobo RA.
Department of Obstetrics and Gynecology, University of Southern California School of Medicine, Los Angeles.
Skin 5 alpha-reductase activity is the major factor influencing the manifestation of androgen excess. Although oral contraceptives have been useful for the treatment of androgen excess, little is known of the independent effects of the various progestins and estrogens on inhibition of skin 5 alpha-reductase activity. We incubated minces of normal genital and pubic skin with physiologic concentrations of 3H-testosterone to assess 5 alpha-reductase activity by its conversion to 3H-dihydrotestosterone.
In separate experiments, 5 alpha-reductase activity was assessed before and after the addition of progesterone, medroxyprogesterone acetate, levonorgestrel, norethindrone, 17 beta-estradiol, and ethinyl estradiol.
Progesterone, levonorgestrel, and norethindrone demonstrated 97 +/- 5.3%, 47.9 +/- 6.3%, and 59 +/- 4.6% inhibition, respectively, of genital skin 5 alpha-reductase activity at 10(-4) mol/L (P less than .01).
Medroxyprogesterone acetate, however, failed to affect 5 alpha-reductase activity at similar doses. Estradiol exhibited 40.8 +/- 14.2% inhibition at 10(-4) mol/L (P less than .01), whereas ethinyl estradiol at concentrations from 10(-8) to 10(-4) mol/L failed to inhibit 5 alpha-reductase activity.
We conclude that progesterone and the 19-nor-derivatives inhibit 5 alpha-reductase activity at high doses, whereas medroxyprogesterone acetate does not. Therefore, the 19-nor-progestin component may expand the usefulness of oral contraceptives in the treatment of hirsutism by an inhibitory action on skin 5 alpha-reductase activity.
Spironolactone for Hirsutism
2) http://www.ncbi.nlm.nih.gov/pubmed/12749435
Fertil Steril. 2003 Apr;79(4):942-6. Use of cyproterone acetate, finasteride, and spironolactone to treat idiopathic hirsutism. Lumachi F, Rondinone R. Department of Surgical and Gastroenterological Sciences, University of Padua, School of Medicine, Padova, Italy.
To compare the effectiveness of cyproterone acetate, finasteride, and spironolactone in the treatment of idiopathic hirsutism.
DESIGN: Prospective randomized clinical study.
SETTING:University hospital.
PATIENT(S): Forty-one women (median age, 21 years [range, 18-34 years]) with idiopathic hirsutism who had requested to use an oral contraceptive.
INTERVENTION(S): Patients were randomly assigned to receive cyproterone acetate (12.5 mg/d for the first 10 days of the cycle), finasteride (5 mg/d), or spironolactone (100 mg/d) for 12 months. Follow-up was done at the end of therapy.
MAIN OUTCOME MEASURE(S): Ferriman-Gallwey score before treatment, at 6 and 12 months of treatment, and 1 year after the end of treatment, and androgenic profile before and after treatment.
RESULT(S): At the end of therapy, the Ferriman-Gallwey score decreased by 38.9%, 38.6%, and 38.5% in patients who used cyproterone acetate, finasteride, and spironolactone, respectively. One year after therapy, the Ferriman-Gallwey score of patients who used spironolactone was significantly lower (6.74 +/- 1.41) than that of patients who used either cyproterone acetate (7.92 +/- 1.08), or finasteride (9.08 +/- 0.99). The androgenic profile did not change significantly during treatment.
CONCLUSION(S): In patients with idiopathic hirsutism, the short-term results of treatment with cyproterone acetate, finasteride, and spironolactone are similar, but spironolactone is effective for a longer time.
Metformin Decreases Insulin, and decreases Testosterone
3) http://sites.csn.edu/science/Biology/Polycystic ovary syndrome.pdf
N Engl J Med. 1996 Aug 29;335(9):617-23.
Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. (using Metformin) Nestler JE, Jakubowicz DJ. Department of Internal Medicine, Medical College of Virginia, Virginia Commonwealth University, Richmond, VA 23298-0111,
Insulin resistance and increased ovarian cytochrome P450c17 alpha activity are both features of the polycystic ovary syndrome. P450c17 alpha, which is involved in androgen biosynthesis, has both 17 alpha-hydroxylase and 17,20-lyase activities. Increased activity of this enzyme results in exaggerated conversion of progesterone to 17 alpha-hydroxyprogesterone in response to stimulation by gonadotrophin. We hypothesized that hyperinsulinemia stimulates ovarian P450c17 alpha activity.
METHODS: We measured fasting serum steroid concentrations and the response of serum 17 alpha-hydroxyprogesterone to leuprolide, a gonadotrophin-releasing hormone agonist, and performed oral glucose-tolerance tests before and after oral administration of either metformin (500 mg three times daily) or placebo for four to eight weeks in 24 obese women with the polycystic ovary syndrome.
RESULTS: In the 11 women given metformin, the mean (+/- SE) area under the serum insulin curve after oral glucose administration decreased from 9303 +/- 1603 to 4982 +/- 911 microU per milliliter per minute (56 +/- 10 to 30 +/- 6 nmol per liter per minute) (P = 0.004).
This decrease was associated with a reduction in the basal serum 17 alpha-hydroxyprogesterone concentration from 135 +/- 21 to 66 +/- 7 ng per deciliter (4.1 +/- 0.6 to 2.0 +/- 0.2 nmol per liter) (P = 0.01) and a reduction in the leuprolide-stimulated peak serum 17 alpha-hydroxyprogesterone concentration from 455 +/- 54 to 281 +/- 52 ng per deciliter (13.7 +/- 1.6 to 8.5 +/- 1.6 nmol per liter) (P = 0.01). The serum 17 alpha-hydroxyprogesterone values increased slightly in the placebo group.
In the metformin group, the basal serum luteinizing hormone concentration decreased from 8.5 +/- 2.2 to 2.8 +/- 0.5 mlU per milliliter (P = 0.01), the serum free testosterone concentration decreased from 0.34 +/- 0.07 to 0.19 +/- 0.05 ng per deciliter (12 +/- 3 to 7 +/- 2 pmol per liter) (P = 0.009), and the serum sex hormone-binding globulin concentration increased from 0.8 +/- 0.2 to 2.3 +/- 0.6 microgram per deciliter (29 +/- 7 to 80 +/- 21 nmol per liter) (P < 0.001). None of these values changed significantly in the placebo group.
CONCLUSIONS: In obese women with the polycystic ovary syndrome, decreasing serum insulin concentrations with metformin reduces ovarian cytochrome P450c17 alpha activity and ameliorates hyperandrogenism.
the administration of metformin reduced the serum insulin concentration during fasting and the insulin response to oral glucose administration.
Concomitantly, ovarian cytochrome P450c17a activity decreased, as demonstrated by a substantial reduction in the response of serum 17a-hydroxyprogesterone to the administration of leuprolide (to increase luteinizing hormone secretion). The reduction in P450c17a activity was accompanied by a decline in the serum free testosterone concentration.
Clomid
4) www.advancedfertility.com/clomid-pcos-treatment.htm
What is the process for taking Clomid?
Establishing “Day One” of the Menstrual Period
•Day one is counted as the first day of menstrual bleeding
•The period can be from a spontaneous menstrual period or from a period induced with a progestin medication such as Provera (medroxyprogesterone acetate) Inducing a Period (if needed)
•In order to induce a period, Provera is given for 5 to 10 days at a dose of 5 or 10mg daily
•The period usually starts within 2-7 days after the last Provera pill is taken Starting Clomid
•Clomid is started early in the menstrual cycle and is taken for five days either from cycle days 3 through 7, or from day 5 through 9
•Clomid is usually started at a dose of one tablet (50mg) daily – taken any time of day Increasing Clomid Dosage (if needed)
•If the patient does not ovulate on the starting dose, Provera is often given to induce a period – then a 100mg dose of Clomid is tried
•That means that a woman taking Clomid on days 5-9 will often ovulate on about day 16-20 of the cycle
•However, there is significant variation in how long it takes ovulate using Clomid. Some will ovulate much later – as late as two or three weeks after the last clomiphene tablet. ——————————————————————————–
How long should I try Clomid before moving on to other treatment options?
There is no set number of cycles of Clomid that should be done before moving on to other fertility treatments. Several variables are involved in the decision about moving on to more aggressive therapy.
No Ovulation Results After increasing Clomid Dosage
•If a woman is not ovulating on a low dose of Clomid, the dose should be increased •If not ovulating at 150 mg then other therapies should be attempted Female age and Clomid treatment and when to be more aggressive
•Relatively fewer cycles should be done with an older female partner
•Clomid probably should not be used at all if the female age is 40 or older because of the significantly reduced fertility potential
•Women 38 or older should probably start fertility treatment with a fertility specialist – rather than with their gynecologist
•If the female is under 38 years old and the sperm is good then usually 3-6 months of Clomid cycles (with good ovulation) are often tried
————————————————————–
5) http://www.advancedfertility.com/clomid-metformin-pcos.htm
http://www.advancedfertility.com/clomid-pcos-treatment.htm
PClomid and Metformin for PCOS Glucophage Plus Clomiphene for Fertility Treatment and Pregnancy with Polycystic Ovarian Syndrome author Richard Sherbahn MD
Metformin and Clomid Use with PCOS
•Women who do not ovulate often have polycystic ovarian syndrome, or PCOS •Clomid, also called Serophene, or clomiphene citrate is the most commonly used medication to try to induce ovulation in women that do not ovulate on their own •Metformin (Glucophage) is an oral medication that is mainly used to treat diabetes •However, it can also be used with PCOS to help women ovulate and achieve pregnancy.
•Some women with PCOS that do not ovulate when treated with Clomid or metformin alone could ovulate and have success when glucophage and Clomid are used together Metformin alone for PCOS
A relatively small percentage of women that do not ovulate regularly and have polycystic ovarian disease will ovulate regularly and become pregnant from treatment with metformin alone. For those women that are taking metformin for PCOS and are not achieving pregnancy, a reasonable option is to add Clomid to the metformin treatment
An Alternative to Injectable FSH Medications, or IVF
Using clomiphene and metformin together can be beneficial for the women with polycystic ovarian syndrome. If they can ovulate and get pregnant with this medication combination, they can avoid the more expensive and invasive treatments such as using injectable FSH medications or in vitro fertilization for PCOS.
Side Effects of Glucophage / Metformin and Clomid for PCOS
About one fourth of women taking metformin have gastrointestinal side effects such as abdominal discomfort, diarrhea and nausea. We do not know of any serious complications from metformin treatment of PCOS.
•Details about side effects of Clomid Treatment Protocol for for Taking Metformin and Clomid to Induce Ovulation
The benefit of metformin on ovulation in women with polycystic ovaries is not seen right away. There is some benefit starting about a month after beginning metformin.
•Metformin has a more substantial benefit for fertility when the woman has been taking it for at least 60 to 90 days.
Metformin Dosing
Metformin is taken in a dose that the woman can tolerate. Most people can tolerate 500 mg three times daily, if they build up to that dose gradually.
•We usually start metformin at 500 mg once daily, then increase to 500 mg twice a day after one week, then to 500 mg three times daily after another week.
•If the three times daily dose cannot be tolerated due to side effects, we remain on the twice-daily dose.
Clomid Dosing
•Clomiphene is usually started at a dose of one tablet (50 mg) daily for five days
•It is started early in the menstrual cycle, usually either days 3 – 7 or days 5 – 9 •Women that have very irregular cycles usually need to have a period induced with a progestin medication such as Provera
•If 50 mg of clomiphene per day does not result in ovulation, we increase the dose in the next cycle to 100 mg per day
•If there is not ovulation at 100 mg, we either try 150 mg per day – or we give up on Clomid and move on to other fertility treatments
————————————————————————-
Clomid for PCOS Metformin Dexamethazone Induction of Ovulation
2006 Egypt
7) www.ncbi.nlm.nih.gov/pubmed/16543255
Hum Reprod. 2006 Jul;21(7):1805-8. Epub 2006 Mar 16.
Clomiphene citrate and dexamethazone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study. Elnashar A, Abdelmageed E, Fayed M, Sharaf M. Department of Obstetrics and Gynecology, Benha University Hospital, Benha, Egypt.
The aim of this work was to evaluate the efficacy of adding dexamethazone (DEX) (high dose, short course) to clomiphene citrate (CC) in CC-resistant polycystic ovary syndrome (PCOS) with normal dehydroepiandrosterone sulphate (DHEAS) in induction of ovulation.
METHODS: Eighty infertile women with CC-resistant PCOS were randomly assigned into two groups.
Group I: Clomiphene citrate 100 mg/day was given from day 3 to day 7 of the cycle and DEX 2 mg/day from day 3 to day 12 of the cycle.
Group II: Same protocol of CC combined with placebo (folic acid tablets) was given from day 3 to day 12 of the cycle.
The main outcome was ovulation. Secondary measures included number of follicles >18 mm endometrial thickness and pregnancy rate. Ovarian follicular response was monitored by transvaginal ultrasound. HCG 10,000 U was given when at least one follicle measured 18 mm, and timed intercourse was advised.
RESULTS: There were no statistically significant differences between groups as regards age, duration of infertility, BMI, waist-hip ratio (WHR), menstrual pattern, hirsutism, serum DHEAS or day of HCG administration.
The mean number of follicles>18 mm at the time of HCG administration and the mean endometrial thickness were significantly higher in the DEX group than in the placebo group (P<0.05).
Similarly, there were significantly higher rates of ovulation (75 versus 15%) (P<0.001) and pregnancy (40 versus 5%) (P<0.05) in the DEX group.
Dexamethazone was very well tolerated as no patients complained of any side effect. There was a significant difference between the responders and non-responders in the presence of oligomenorrhea, amenorrhea or hirsutism.
CONCLUSION: Induction of ovulation by adding DEX (high dose, short course) to CC in CC-resistant PCOS with normal DHEAS is associated with no adverse anti- estrogenic effect on the endometrium and higher ovulation and pregnancy rates in a significant number of patients. Induction with DEX appears to be independent on age, period of infertility, BMI or WHR.
2002- IRAN
8) www.ncbi.nlm.nih.gov/pubmed/12413984
Fertil Steril. 2002 Nov;78(5):1001-4.
Use of dexamethasone and clomiphene citrate in the treatment of clomiphene citrate-resistant patients with polycystic ovary syndrome and normal dehydroepiandrosterone sulfate levels: a prospective, double-blind, placebo-controlled trial.
Parsanezhad ME, Alborzi S, Motazedian S, Omrani G. Source Division of Infertility, Department of Obstetrics and Gynecology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
To evaluate the effects of short-course administration of dexamethasone (DEX) combined with clomiphene citrate (CC) in CC-resistant patients with polycystic ovary syndrome (PCOS) and normal DHEAS levels.
DESIGN: Prospective, double-blind, placebo-controlled, randomized study.
SETTING: Referral university hospitals. PATIENT(S): Two hundred thirty women with PCOS and normal DHEAS who failed to ovulate after a routine protocol of CC. INTERVENTION(S): The treatment group received 200 mg of CC from day 5 to day 9 and 2 mg of DEX from day 5 to day 14 of the menstrual cycle. The control group received the same protocol of CC combined with placebo.
MAIN OUTCOME MEASURE(S): Follicular development, hormonal status, ovulation rate, pregnancy rate.
RESULT(S): Mean follicular diameters were 18.4124 +/- 2.4314 mm and 13.8585 +/- 2.0722 mm for the treatment and control groups, respectively. Eighty-eight percent of the treatment group and 20% of the control group had evidence of ovulation. The difference in the cumulative pregnancy rate in the treatment and control groups was statistically significant.
CONCLUSION(S): Hormonal levels, follicular development, and cumulative pregnancy rates improved with the addition of DEX to CC in CC-resistant patients with PCOS and normal DHEAS. This regimen is recommended before any gonadotropin therapy or surgical intervention.
——–
1992- Louisiana
9) www.ncbi.nlm.nih.gov/pubmed/1564704
J Reprod Med. 1992 Mar;37(3):215-8.
Clomiphene-dexamethasone treatment of clomiphene-resistant women with and without the polycystic ovary syndrome. Singh KB, Dunnihoo DR, Mahajan DK, Bairnsfather LE. Department of Obstetrics and Gynecology, Louisiana State University School of Medicine, Shreveport 71130.
We used clomiphene and dexamethasone in 40 infertile women to treat chronic anovulation resistant to the use of clomiphene alone. Eighteen (45%) of the women had the polycystic ovary (PCOS) syndrome; the remaining 22 (55%) had clomiphene-resistant anovulation from idiopathic causes. Both groups of women were similar in regard to age, parity, duration of infertility and absence of other causes of infertility besides chronic anovulation.
Ovulation could be induced in approximately 90% of the women in each group. Altogether, 19 of 36 women (52.8%) conceived without any side effects or complications. The cumulative probability of conception at nine months of treatment was 87.5% in PCOS patients and 46% in the non-PCOS group.
Clomiphene plus dexamethasone was highly effective in the treatment of clomiphene-resistant anovulation associated with infertility in women with and without the PCO syndrome.
————————————————
1980- Germany
10) www.ncbi.nlm.nih.gov/pubmed/7445819
Zentralbl Gynakol. 1980;102(12):645-50. [Combined clomiphene-dexamethasone therapy in cases with resistance to clomiphene (author’s transl)]. [Article in German] Lisse K. Abstract
Combined clomiphene-dexamethasone treatment was applied to 13 patients with clomiphene-resistant anovulation which had been caused by androgenic hyperactivity of ovarian or adrenal origin.
The women, following induced withdrawal bleeding, received
200 mg/die clomiphene over five days, from the fifth through the ninth days of their cycles,
plus 2 mg/die dexamethasone over ten days, from the fifth to 14th days of cycle, after they had failed to conceive in response to clomiphene alone administered in rising doses up to 200 mg/die.
Nine pregnancies were successfully induced by that treatment. Two of them ended in abortion and one in premature twin delivery. Combined clomiphene-dexamethasone treatment is recommended for clomiphene-resistant cases with ovarian or adrenal hyperandrogenism, particularly for patients with polycystic ovaries (Stein- Leventhal syndrome).
————————————–
1982
11) www.ncbi.nlm.nih.gov/pubmed/6214735
Obstet Gynecol. 1982 Oct;60(4):497-501. Clomiphene and dexamethasone in women unresponsive to clomiphene alone. Lobo RA, Paul W, March CM, Granger L, Kletzky OA.
Twelve oligomenorrhic women with polycystic ovary syndrome (PCO) in whom clomiphene (250 mg daily for 5 days) and 10,000 IU human chorionic gonadotropin had failed to induce ovulation were treated with clomiphene and dexamethasone. Eight of the 12 women underwent complete hormonal assessment during treatment. Six of the 12 ovulated and 1 conceived. Serum total and unbound estradiol and testosterone (T), serum dehydroepiandrosterone sulfate (DHEA-S), sex hormone binding-globulin binding capacity (SHBG-BC), luteinizing hormone (LH), follicle-stimulating hormone (FSH) and prolactin (PRL) were measured during clomiphene and dexamethasone therapy. SHBG-BC increased in response to clomiphene whether or not ovulation occurred. After treatment with clomiphene and dexamethasone there was a significant decrease in serum T, unbound T, and DHEA-S 2 weeks after dexamethasone administration, but there were no change in LH, FSH, or PRL. In patients who ovulated after clomiphene and dexamethasone, T and unbound T increased again after clomiphene was begun despite the continuation of dexamethasone. The women who ovulated after clomiphene and dexamethasone treatment had significantly higher pretreatment levels of DHEA-S than those who did not ovulate. Clomiphene and dexamethasone treatment may be beneficial to women who have elevated levels of DHEAS and who fail to ovulate with maximum doses of clomiphene.
———————————————
Clomid for PCOS
1989
12) www.ncbi.nlm.nih.gov/pubmed/2490173
Rev Chil Obstet Ginecol. 1989;54(2):94-7.
[Treatment of anovulation in polycystic ovary syndrome with clomiphene citrate in 10-day cycles]. [Article in Spanish] Porcile A, Venegas E, Gallardo E.Abstract The efficacy of a new schedule for the use of Clomiphene citrate (50 mg daily, during the first ten days of the cycle) is reported, in relation to ovulation induction and fertility. We treated 41 patients with the syndrome of polycystic ovary, who were anovulant and desired fertility. Ovulation was successfully induced in 35 of them (85.4%). Pregnancy was achieved in 28 patients (68.3%).
In 87.5% of those becoming pregnant, gestations was achieved with less than 6 months of treatment. Our results are equally good or better than those reported in the literature with conventional treatment. Pregnancies obtained were normal. No congenital malformations were observed. There were only two abortions, spontaneous. No patient presented ovarian hyperstimulation syndrome. This new schedule is recommended because it is effective and it may give less complications. Also, it is not necessary to increase progressively the dose of clomiphene citrate.
—————————-
2009 Clomid Compared to Metformin for PCOS
13) www.ncbi.nlm.nih.gov/pubmed/18321486
Fertil Steril. 2009 Feb;91(2):514-21. Epub 2008 Mar 5.
Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial.
Zain MM, Jamaluddin R, Ibrahim A, Norman RJ.
Source Department of Obstetric and Gynaecology, Alor Setar Hospital, Kedah, Malaysia.
To determine the first-line medication to be used in anovulatory patients with polycystic ovary syndrome (PCOS) for ovulation induction and pregnancy achievement.
DESIGN: Randomized controlled trial.
SETTING: Infertility unit of a public hospital.
PATIENT(S): One hundred fifteen newly diagnosed patients with PCOS based on ESHRE/ASRM criteria.
INTERVENTION(S): These patients were assigned to three groups: group 1 (38 patients) received 500 mg of metformin three times a day; group 2 (39 patients) received clomiphene citrate (CC) at an incremental dose; group 3 (38 patients) received both medications.
MAIN OUTCOME MEASURE(S): Rates of ovulation, pregnancy (PR), and live birth. RESULT(S): The ovulation rate was 23.7% in the metformin group, 59% in the CC group, and 68.4% in the combination treatment group. This was translated into a similar PR and live birth rate, which were higher in the CC and combination groups compared to the metformin group (PR: 7.9%, 15.4%, and 21.1%; live birth rate: 7.9%, 15.4%, and 18.4% in metformin, CC, and combination treatment groups, respectively), although statistically the differences were not significant. There were no multiple pregnancies and the rate of spontaneous first trimester loss was similar to the general population.
CONCLUSION(S): Clomiphene citrate should be the first-line treatment for ovulation induction in anovulatory patients with PCOS. ——————————————————————————
Textbook on Androgen Excess Disorders in Women free online
6) http://www.scribd.com/doc/36327773/Androgen-Excess-Disorders-in-Women
Androgen Excess Disorders in Women: Polycystic Ovary Syndrome, NonClassical CAH and Others … By Ricardo Azziz, John E. Nestler, Didier Dewailly
—————————–
PCOS – Hirsutism – Useful testing day 19 Follicular phase
17 hydroxy progesterone
ACTH
24 hour urinary cortisol
Dexamethasone suppression test
——————————————————–
Dexamethasone Reduces Androgen Levels in PCOS patients
2004 NORWAY
14) http://humrep.oxfordjournals.org/content/19/3/529.full
http://humrep.oxfordjournals.org/content/19/3/529.full.pdf
Hum. Reprod. (2004) 19 (3): 529-533.
Six‐ month treatment with low‐dose dexamethasone further reduces androgen levels in PCOS women treated with diet and lifestyle advice, and metformin
E. Vanky1,3,K.Å. Salvesen1 and S.M. Carlsen2 1Department of Obstetrics and Gynecology and 2Section of Endocrinology, Department of Medicine, St Olavs Hospital, University Hospital of Trondheim, Trondheim, Norway.
BACKGROUND: The purpose of this study was to investigate the effect of low‐dose dexamethasone on androgen levels in women with polycystic ovary syndrome (PCOS) treated with diet and lifestyle counselling, and metformin.
METHODS: A prospective, randomized, double blind, placebo‐controlled study was carried out. Thirty‐eight women with PCOS were randomized to either dexamethasone 0.25 mg daily or placebo for 26 weeks. All received diet and lifestyle counselling at inclusion and metformin 850 mg three times daily during the whole study. Main outcome measures were: androgen levels, body mass index (BMI), insulin c‐peptide, fasting glucose and serum lipids. Two‐tailed t‐tests and Pearson’s statistics were used. RESULTS: Compared with the placebo, dexamethasone reduced testosterone by 27%, androstenedione by 21%, dehydroepiandrosterone sulphate by 46% and free testosterone index by 50% in women with PCOS treated with diet and lifestyle advice, and metformin. BMI, fasting glucose, insulin c‐peptide and serum lipid levels were unaffected.
CONCLUSIONS: Six‐month, low‐dose dexamethasone treatment further reduces androgen levels in metformin‐trated PCOS women.
2003 MAYO CLINIC ROCHESTER MINN
15) http://www.jfponline.com/pages.asp?aid=3059
February 2003 · Vol. 15, No. 2 EXAMINING THE EVIDENCE
Dexamethasone-clomiphene citrate (CC) treatment in CC-resistant PCOS patients
DANIEL DUMESIC, MD CONSULTANT, REPRODUCTIVE ENDOCRINOLOGY AND INFERTILITY MAYO CLINIC . ROCHESTER, MINN The question: Does dexamethasone-clomiphene citrate (CC) treatment improve ovulation rates in CC-resistant patients with polycystic ovary syndrome?
Two hundred thirty infertile PCOS patients who failed to ovulate during CC therapy (250 mg/day orally for 5 days) and human chorionic gonadotropin (hCG) administration (10,000 units, intramuscularly) were randomized to CC (200 mg/day, cycle days 5-9) with or without DEX (2 mg/day, cycle days 5-14).
2011 EGYPT Review Article
16) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3039006/
Int J Womens Health. 2011; 3: 25–35.
Published online 2011 February 8. doi: 10.2147/IJWH.S11304
PMCID: PMC3039006
Treatment options for polycystic ovary syndrome
Ahmed Badawy1 and Abubaker Elnashar2
1Department of Obstetrics and Gynecology, Mansoura University, Mansoura, Egypt;
2Department of Obstetrics and Gynecology, Benha University, Benha, Egypt
Glucocorticoids such as prednisone and dexamethasone have been used to induce ovulation. Elnashar et al demonstrated that induction of ovulation by adding dexamethasone (high dose, short course) to CC in CC-resistant PCOS with normal DHEAS is associated with no adverse antiestrogenic effect on the endometrium and higher ovulation and pregnancy rates in a significant number of patients.34
In PCOS patients with high adrenal androgen, low-dose dexamethasone (0.25–0.5 mg) at bedtime can be used.35
In a study of 230 women with PCOS who failed to ovulate with 200 mg of CC for 5 days, addition of 2 mg of dexamethasone from days 5–14 is associated with a higher ovulation rate and cumulative pregnancy rate.36 Enthusiasm for their use is dampened, however, by their potential adverse effects on insulin sensitivity; therefore, prolonged use should be discouraged.
2006 EGYPT
17) humrep.oxfordjournals.org/content/21/7/1805.long
,,,
http://www.ncbi.nlm.nih.gov/pubmed/16543255
Hum Reprod. 2006 Jul;21(7):1805-8. Epub 2006 Mar 16.
Clomiphene citrate and dexamethazone in treatment of clomiphene citrate-resistant polycystic ovary syndrome: a prospective placebo-controlled study.
Elnashar A, Abdelmageed E, Fayed M, Sharaf M.
SourceDepartment of Obstetrics and Gynecology, Benha University Hospital, Benha, Egypt.
BACKGROUND:The aim of this work was to evaluate the efficacy of adding dexamethazone (DEX) (high dose, short course) to clomiphene citrate (CC) in CC-resistant polycystic ovary syndrome (PCOS) with normal dehydroepiandrosterone sulphate (DHEAS) in induction of ovulation.
METHODS:Eighty infertile women with CC-resistant PCOS were randomly assigned into two groups. Group I: Clomiphene citrate 100 mg/day was given from day 3 to day 7 of the cycle and DEX 2 mg/day from day 3 to day 12 of the cycle. Group II: Same protocol of CC combined with placebo (folic acid tablets) was given from day 3 to day 12 of the cycle. The main outcome was ovulation. Secondary measures included number of follicles >18 mm endometrial thickness and pregnancy rate. Ovarian follicular response was monitored by transvaginal ultrasound. HCG 10,000 U was given when at least one follicle measured 18 mm, and timed intercourse was advised.
RESULTS:there were no statistically significant differences between groups as regards age, duration of infertility, BMI, waist-hip ratio (WHR), menstrual pattern, hirsutism, serum DHEAS or day of HCG administration. The mean number of follicles>18 mm at the time of HCG administration and the mean endometrial thickness were significantly higher in the DEX group than in the placebo group (P<0.05). Similarly, there were significantly higher rates of ovulation (75 versus 15%) (P<0.001) and pregnancy (40 versus 5%) (P<0.05) in the DEX group. Dexamethazone was very well tolerated as no patients complained of any side effect. There was a significant difference between the responders and non-responders in the presence of oligomenorrhea, amenorrhea or hirsutism.
CONCLUSION:Induction of ovulation by adding DEX (high dose, short course) to CC in CC-resistant PCOS with normal DHEAS is associated with no adverse anti-estrogenic effect on the endometrium and higher ovulation and pregnancy rates in a significant number of patients. Induction with DEX appears to be independent on age, period of infertility, BMI or WHR.
2003 Germany
18) jcem.endojournals.org/content/88/6/2760.long
http://www.ncbi.nlm.nih.gov/pubmed/12788885
J Clin Endocrinol Metab. 2003 Jun;88(6):2760-6.
Beyond adrenal and ovarian androgen generation: Increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome.
Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W.
Source Department of Medicine, Endocrine and Diabetes Unit, University of Würzburg, 97080 Würzburg, Germany.
Abstract Hyperandrogenism, a main clinical feature of polycystic ovary syndrome (PCOS), is thought to result from enhanced ovarian and adrenal androgen generation. To investigate the contribution of peripheral steroidogenesis, we used an oral challenge with dehydroepiandrosterone (DHEA) and analyzed its downstream conversion toward androgens in eight women with PCOS (age, 20-32 yr; body mass index, 20-41 kg/m(2)) and eight healthy women matched for age and body mass index. They underwent frequent serum sampling and urine collection for 8 h on three occasions: at baseline, and after 4 d of dexamethasone (Dex; 4 x 0.5 mg/d), followed by ingestion of 100 mg DHEA or placebo. Dex induced similar significant suppression of circulating steroids in both groups. The oral DHEA challenge led to similar significant increases in the area under the concentration-time curve (0-8 h after Dex) of serum DHEA, DHEA sulfate, androstenedione, and testosterone. However, after oral DHEA, PCOS women had significantly higher increases in serum 5 alpha-dihydrotestosterone (P < 0.01), its main metabolite androstanediol glucuronide (P < 0.05), and the 5 alpha-reduced urinary androgen metabolite androsterone (P < 0.05). PCOS women also had significantly higher baseline excretion of 5 alpha-reduced glucocorticoid (P < 0.01) and mineralocorticoid metabolites (P < 0.05). Taken together, these data indicate enhanced peripheral 5 alpha-reductase activity in PCOS. Thus, not only ovary and adrenal, but also liver and peripheral target tissues, significantly contribute to steroid alterations in PCOS.
UK
19) jcem.endojournals.org/content/88/6/2634.full
The Value of the Low-Dose Dexamethasone Suppression Test in the Differential Diagnosis of Hyperandrogenism in Women Gregory A. Kaltsas, Andrea M. Isidori, Blerina P. Kola, Rob H. Skelly, Shern L. Chew, Paul J. Jenkins, John P. Monson, Ashley B. Grossman and G. Michael Besser Department of Endocrinology, St. Bartholomew’s Hospital, London ECIA 7BE, United Kingdom
BOSNIA
20) www.healthmedjournal.110mb.com/files/vol03-no4.pdf#page=108
Therapeutic effects of metformin, rosiglitazone, and dexamethasone in reproductive women with PCOS Hajder M.1, Hajder E.2, Kudumovic A.3 1 University Clinical Center Tuzla, Internal clinic, department of Endocrinology, Bosnia and Herzegovina,
2 University Clinical Center Tuzla, Gynecology and Obstetrics clinic, Bosnia and Herzegovina, 3 Medical Faculty of the University of Sarajevo, Bosnia and Herzegovina
Maria New
21) jcem.endojournals.org/content/91/11/4205.abstract
The Journal of Clinical Endocrinology & Metabolism November 1, 2006 vol. 91 no. 11 4205-4214 . Nonclassical 21-Hydroxylase Deficiency Maria I. New Department of Pediatrics, Mount Sinai School of Medicine, New York, New York 10029
Context: Nonclassical congenital adrenal hyperplasia (CAH) owing to steroid 21-hydroxylase deficiency (NC21OHD) is the most frequent of all autosomal recessive genetic diseases, occurring in one in 100 persons in the heterogeneous New York City population. NC21OHD occurs with increased frequency in certain ethnic groups, such as Ashkenazi Jews, in whom one in 27 express the disease. NC21OHD is underdiagnosed in both male and female patients with hyperandrogenic symptoms because hormonal abnormalities in NC21OHD are only mild to moderate, not severe as in the classical form of CAH. Unlike classical CAH, NC21OHD is not associated with ambiguous genitalia of the newborn female.
Main Outcome Measures: The hyperandrogenic symptoms include advanced bone age, early pubic hair, precocious puberty, tall stature, and early arrest of growth in children; infertility, cystic acne, and short stature in both adult males and females; hirsutism, frontal balding, polycystic ovaries, and irregular menstrual periods in females; and testicular adrenal rest tissue in males.
Conclusions: The signs and symptoms of hyperandrogenism are reversed with dexamethasone treatment.
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/ disc.yourwebapps.com/Indices/244124.html
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
disc.yourwebapps.com/Indices/244161.html
disc.yourwebapps.com/Indices/244163.html
Disclaimer click here: www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article:
http://jeffreydach.com/2012/06/20/pcos-part-two-clomid-and-metformin-by-jeffrey-dach-md.aspx
Copyright (c) 2012-2013 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.
PCOS the Hidden Epidemic Part Two by Jeffrey Dach MD - Jeffrey Dach MD January 24, 2014 at 5:44 PM
[…] PCOS The Hidden Epidemic Part Two by Jeffrey Dach MD. Cindy, a 34 year old flight attendant had been taking birth control pills for 17 years because of PCOS (Polycystic Ovary Syndrome).. Cindy came for an office visit because she wanted a treatment that would restore normal menstrual cycles, fertility, as well eliminate the excess facial hair, acne and other androgenic effects of elevated testosterone levels….For More Click Here […]
Myo-Inositol for PCOS and Hashimotos - Jeffrey Dach MD February 18, 2014 at 7:50 AM
[…] PCOS Part Two […]
PCOS Part One by Jeffrey Dach MD - Jeffrey Dach MD April 14, 2014 at 9:05 AM
[…] This article is Part One of a series, For Part Two, Click Here. […]