 Clomiphene Clomid Adverse Side Effects Part Three
Clomiphene Clomid Adverse Side Effects Part Three
This is part three of a series,
Click Here for Part One
Click Here for Part Two
The off-label use of clomiphene tablets in young males to relieve symptoms of low testosterone is becoming more popular because it preserves fertility, and is less expensive and more convenient than testosterone injections. Best results are obtained in males of child bearing ages (30 – 50 yrs of age) with hypothalamic dysfunction, (ie. low FSH/LH, and good testicular reserve which can respond to FSH/LH stimulation) Before starting clomiphene, the patient should be conversant with the adverse effects of treatment.
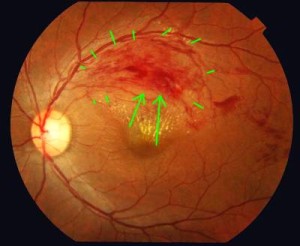
Above left image: Retina of the left eye shows retinal vein occlusion resulting in hemorrhages and exudates marked by Green Arrows, courtesy of wikimedia commons.
Review of the Medical Literature on Adverse Effects of Clomiphene
Dr Viola from Capetown South Africa reviewed the medical literature on common adverse effects of clomiphene treatment, mostly in women. She found 35 articles on the topic and published her report in 2011. She found that blurred visual and other visual effects were most commonly reported about 1.5% of the time.(1)
“Visual adverse events with the use of CC in clinical studies was reported as 1.5%. These include blurred vision, photophobia, diplopia, scotomata, phosphenes and periphlebitis.”(1)
Dr. Viola also reported on central retinal vein occlusion as an adverse effect of clomipene.(1)
Central Retinal Vein Occlusion in a Male
Although a number of reports of retinal vein occlusion have been reported in women using clomiphene (Clomid) as a fertility treatment to induce ovulation, only one is reported in a 35 year old male undergoing fertility treatment with Clomiphene. This male patient had an underlying inherited thrombophilia, with Factor V Leiden and MTHFR polymorphism.(2) His retinal vein occlusion, due to thrombosis of the vein, was treated with Plavix (clopidogrel) with recovery. Dr Politou suggests young men should be screened for Factor V Leiden before starting Clomiphene.
Visual Disturbances-Blurred Vision
Dr. Purvin from Methodist Hospital in Indiana reported on visual disturbances from Clomiphene in three women undergoing fertility treatment for 4-12 months.(9)
” All three patients experienced prolonged afterimages (palinopsia), shimmering of the peripheral field, and photophobia while undergoing treatment with clomiphene. The results of the neuro-ophthalmologic examination and electrophysiologic studies were normal in all three patients. Unlike previously reported cases, visual symptoms did not resolve on cessation of treatment. Patients remain symptomatic from 2 to 7 years after discontinuing treatment with the medication.”(9)
“The most common side-effect is without question blurred vision; while this may be a problem for some Clomid users it will dissipate once use is discontinued. “(9)
One case of bilateral uveitis (inflammation of the eye) was described in a 30 year old female on clomiphene.(8) One case of elevated liver enzymes was reported (7)
Psychotic Reaction
In women undergoing fertility treatment with prolonged high dose Clomiphene, various central nervous system adverse effects have been reported including induction of psychosis, nervousness, sleeplessness, headaches, visual disturbances, vertigo.(3,4,5)
Psychological Effects on Mood
In a 2005, large self-reported survey of women on Clomiphene and HMG adverse psychological effects were reported including, Irritability, mood swings, and feeling down.(6)
Estrogen and HyperCoagulable State
Most adverse effects of clomiphene are reported for female fertility treatment using high dose Clomiphene. I would speculate that most of the adverse effects are due to high estrogen levels known to cause hypercoagulable state.(10,11)
Anastrazole to Keep Estrogen Levels Low and Prevent Hypercoagulation.
Adverse effects from off-label use of clomiphene is less commonly reported in the medical literature in males than in females, possibly because of the lower dosage used. Females dosage is 50-200 mg per day, while for males, we use the lower dosage of 12.5 mg every other day up to 25 mg per day.
Estrogen and Hypercoagulable State
For female patients undergoing fertility treatment to induce ovulation, elevated estrogen is part of the desired outcome. Estrogen levels may rise by a factor of one hundred (100 times higher than pre-treatment levels), inducing a hypercoagulable state.(10,11) Quite the opposite in males, clomiphene induced elevated estrogen is an undesirable side effect, preventable with an aromatase inhibitor (anastrazole).
In males, central retinal vein occlusion with underlying inherited thrombophila mutation has been reported.(2) The authors say the causal mechanism is unknown. I would differ, and suggest the cause is high estrogen induced by clomiphene stimulation. High estrogen causes a hypercoagulable state.(10,11) An aromatase inhibitor (anastrazole) prevents the high estrogen level, and therefore prevent the rare thrombotic complication, such as the retinal vein occlusion in the inherited thrombophilia male patient. (2) I would suggest a prudent practice would be to use an aromatase inhibitor with the clomiphene.
Articles with Related Interest:
This is part three of a series,
Click Here for Part One
Click Here for Part Two
Links and References:
Retinal Vein Occlusion and other visual disturbances
1) http://www.karger.com/Article/
http://content.karger.com/
Gynecol Obstet Invest 2011;71:73–76 Fulltext PDF (120 Kb)
Review Association between Clomiphene Citrate and Visual Disturbances with Special Emphasis on Central Retinal Vein Occlusion: A Review
Viola M.I.a · Meyer D.b · Kruger T. Department of Obstetrics and Gynecology, Reproductive Medicine Unit, Stellenbosch University and Vincent Pallotti Hospital, Cape Town,bDivision of Ophthalmology, Stellenbosch University, Tygerberg, South Africa
To determine whether clomiphene citrate (CC) can be implicated as a cause for central retinal vein occlusion (CRVO) and other visual disturbances.
For this systematic review, we performed a search of the following databases: PubMed (1976 to November 2009), Medline Plus 2009, Cochrane Library (1996 to November 2009), Google and Google Scholar (1996 to November 2009).
Thirty-five relevant titles (25 full papers and 10 abstracts) were identified and read by authors. No review has been published in the literature. The publications included describe adverse effects with clomid and selective estrogen receptor modulators and in particular visual disturbances. The population consisted of infertility patients under ovulation induction with CC. The main outcome measures were loss of vision due to CRVO and other visual changes.
RESULTS: CC may predispose to CRVO, but further trials are clearly needed in this area.
CONCLUSION:Physicians should be aware of the potential risk of CC, especially in patients with associated risk factors for CRVO. If visual disturbances occur, therapy should be terminated and the patient referred for specialist ophthalmic care.
Retinal vein occlusion is due to thrombosis of retinal veins (central, hemi or branch) and has been associated with cardiovascular risk factors [2] . However, the pathogenesis and management of this disorder remains somewhat
of an enigma [3] . It is a complication not commonly seen by the gynecologists, but they should be aware of the condition in fertility patients undergoing ovulation induction with clomiphene citrate (CC).
Other CC adverse effects are [4–6] : ovarian enlargement, vasomotor flashes,
nausea, vomiting, breast discomfort, headache, abnormal
vaginal bleeding, visual symptoms, weight gain, shortness of breath; long term use may increase the risk of ovarian cancer and multiple pregnancy [7] .
Other less common side effects have been described: acute pancreatitis without hypertriglyceridemia [8] , myocardial infarction[9] , hypertriglyceridemia [10] , deep vein thrombosis[11] , pulmonary embolism [12] .
The incidence of visual adverse events with the use of CC in clinical studies was reported as 1.5%. These include blurred vision, photophobia, diplopia, scotomata, phosphenes [6] and periphlebitis [18] .
Another case of CRVO was reported due to CC in a 35-year-old man treated for infertility. The patient was heterozygous for the factor V Leiden mutation and the
MTHFR polymorphism, but probably the event was precipitated due to the presence of CC [24] .
Central Retinal Vein Occlusion – Male Factor V leiden carrier
2) http://www.ncbi.nlm.nih.gov/
Genet Test Mol Biomarkers. 2009 Apr;13(2):155-7.
Central retinal vein occlusion secondary to clomiphene treatment in a male carrier of factor V Leiden. Politou M, Gialeraki A, Merkouri E, Travlou A, Baltatzis S.
Abstract We report a case of a 35-year-old previously healthy man treated with clomiphene for infertility, who presented with blurred vision in his left eye due to ocular vein occlusion as documented by fluorescein angiography. The patient was heterozygous for the factor V Leiden (FV Leiden) mutation and for the 1298 A-C polymorphism of the methylene-tetrahydrofolate reductase (MTHFR) gene. He was treated with clopidogrel (plavix) and is now free of symptoms. Because congenital thrombophilia is a moderate risk factor for central retinal vein occlusion and the administration of clomiphene may trigger this process, we recommend screening of young patients for FV Leiden before clomiphene treatment.
Other side effects in Women on Clomid
Psychotic Reaction-
central nervous symptoms (nervousness, sleeplessness, headaches, visual disturbances, vertigo)
3) http://www.ncbi.nlm.nih.gov/
http://humrep.oxfordjournals.
Human Reproduction vol.12 no.4 pp.706–707, 1997
Clomiphene citrate as a possible cause of a psychotic reaction during infertility treatment Department of Obstetrics and Gynecology, DRK-Kliniken Westend, Berlin, Germany.
Secondary side-effects often occur in women undergoing hormonal stimulation treatment with clomiphene citrate. In general 10.4% of women experience hot flushing, 5.5% have complaints caused by enlargement of the ovaries and 3.5% experience central nervous symptoms (nervousness, sleeplessness, headaches, visual disturbances, vertigo).
During ovarian stimulation with clomiphene citrate for in-vitro fertilization, a 32 year old patient developed psychotic symptoms, commencing 3 days after initiation of treatment. Hospitalization in the psychiatric ward became necessary when severe formal and rational thought disturbances arose together with perceptory and sensory delusions. Under neuroleptic treatment the symptoms improved. Nevertheless, follow-up psychiatric care on an outpatient basis was deemed necessary. The infertility treatment was continued with human menopausal gonadotrophin stimulation. Psychiatric instability occurred neither at this point nor during the 2 year follow-up observation period. Both an exogenous psychosis (ICD F23.9) as well as the exacerbation of an endogenous psychosis (ICD F29) may be considered for the differential diagnosis. The stimulation with clomiphene citrate in connection with the physical and psychic stress of the infertility therapy can be regarded as the trigger factor. For patients with evidence of psychiatric illness in their case history, ovulation-inducing substances such as clomiphene citrate should be implemented with particular care.
4) http://www.ncbi.nlm.nih.gov/
Dtsch Med Wochenschr. 1990 Jun 15;115(24):936-9.
[Psychotic illness during treatment with clomifen].
[Article in German] Kapfhammer HP, Messer T, Hoff P.
psychiatrische Klinik und Poliklinik, Universität München.
Psychotic illness recurred in a 25-year-old manic-depressive woman during anti-infertility treatment with clomiphene citrate. The symptoms of this episode (disturbance of consciousness, psychomotoric abnormalities, paranoid-hallucinatory syndrome with transient neurological dysfunctions) differed significantly from previous and subsequent psychotic patterns. No organic cause other than treatment with clomiphene could be discovered despite intensive diagnostic efforts. The pathogenetic role of clomiphene is supported by comparable findings in certain post-partum psychoses. Women with a history of psychiatric disorders are especially at risk.
5) http://www.ncbi.nlm.nih.gov/
Can Med Assoc J. 1982 January 15; 126(2): 118.
Clomiphene citrate as a possible cause of psychosis.
F. E. Cashman and R. Sheppard
6) http://www.ncbi.nlm.nih.gov/
J Psychosom Obstet Gynaecol. 2005 Jun;26(2):93-100.
Psychological side-effects of clomiphene citrate and human menopausal gonadotrophin. Choi SH, Shapiro H, Robinson GE, Irvine J, Neuman J, Rosen B, Murphy J, Stewart D. Women’s Health Program, University Health Network, University of Toronto, Ontario, Canada.
This study evaluated the psychological side-effects of clomiphene citrate (CC) and hMG in women undergoing fertility treatment.
METHOD: This study was a cross-sectional, self-report survey of 454 women at various stages of treatment for infertility. At the time of study, 139 women had not taken fertility drugs and 315 women had taken one or more cycles of CC or hMG. All subjects were asked to complete the State-Trait Anxiety Inventory (STAI). Women taking CC or hMG were also asked to complete a self-administered questionnaire on the side-effects of their medications.
RESULT(S): In the CC group (n = 162) and hMG group (n = 153), 77.8% (126 of 162) and 94.8% (145 of 153) reported at least one side-effect, respectively. Irritability, mood swings, feeling down, and bloating had high frequencies in both CC and hMG groups, with a higher mean number of side effects reported in the hMG group (4.4 +/- 3.7 for the CC group and 6.8 +/- 3.7 for the hMG group, p < 0.001). There was no significant difference among the CC, hMG and no medication groups for mean state and trait anxiety scores. However, there were significant differences among the three side-effect groups (those who reported 1 to 4, 5 to 7, and more than 7 side-effects) for the mean scores of state (df = 2, F = 8.7, p < 0.001) and trait (df = 2, F = 11.9, p < 0.001) anxiety in women taking fertility drugs.
CONCLUSION(S): Women taking CC or hMG reported high frequencies of psychological side-effects, and should be advised of these before treatment.
Elevated LFT’s
7) http://www.ncbi.nlm.nih.gov/
J Reprod Med. 2007 May;52(5):437-8.
Transaminitis after treatment with clomiphene citrate: a case report.
Mitchell C, Gottlieb L. Department of Obstetrics and Gynecology, University of Washington, Box 356460, Seattle, WA 98195-6460, USA.
Clomiphene citrate is widely used to induce ovulation in infertile women with anovulation. The manufacturer reports transaminitis as a possible side effect, but no case reports were found on a literature search.
A 30-year-old, Somali woman, gravida 3, para 1021, was referred for evaluation after trying unsuccessfully to conceive for 19 months. She was diagnosed with unexplained infertility and prescribed clomiphene citrate for ovulation induction. The patient took the medication in 2 consecutive cycles and each time developed intense right upper quadrant pain. During the second episode she presented to the emergency room with transaminitis. Acute and chronic viral hepatitis were ruled out, and she had not ingested any other medications; ultimately, no other etiology was discovered. The transaminitis resolved over 2 months and, in the absence of further treatment with clomiphene citrate, did not recur.
Transaminitis is a rare complication of treatment with clomiphene citrate. Liver function testing is warranted in patients with new-onset right upper quadrant pain after starting treatment with clomiphene citrate.
Uveititis
8) http://www.ncbi.nlm.nih.gov/
Ocul Immunol Inflamm. 2008 Jan-Feb;16(1):23-4.
Bilateral anterior uveitis associated with clomiphene citrate.Myers TD, Fraunfelder FW. Source Casey Eye Institute, 3375 SW Terwilliger Blvd, Portland, OR 97239-4197, USA.
To report a case of bilateral anterior uveitis associated with ovulation induction therapy using clomiphene citrate.DESIGN:Retrospective case review.A single patient who developed uveitis while taking clomiphene citrate is described.
RESULTS:A 30-year-old woman with polycystic ovary syndrome developed bilateral anterior uveitis during ovulation induction therapy. Results of laboratory studies were normal and the patient was treated with topical therapy. Upon rechallenge with clomiphene 3 months later, she again developed bilateral anterior uveitis.
CONCLUSIONS:Ovulation induction therapy with clomiphene citrate may precipitate uveitis.
Visual Disturbances
9) http://www.ncbi.nlm.nih.gov/
Arch Ophthalmol. 1995 Apr;113(4):482-4.
Visual disturbance secondary to clomiphene citrate. Purvin VA. Source Midwest Eye Institute, Methodist Hospital of Indiana, Indianapolis, USA.
To identify a distinctive constellation of persistent visual abnormalities secondary to treatment with clomiphene citrate.
DESIGN: Description of the clinical findings in three patients with visual disturbance secondary to clomiphene treatment.
SETTING: A neuro-ophthalmology referral center.
PATIENTS: Three women aged 32 to 36 years treated for infertility with clomiphene for 4 to 15 months.
RESULTS: All three patients experienced prolonged afterimages (palinopsia), shimmering of the peripheral field, and photophobia while undergoing treatment with clomiphene. The results of the neuro-ophthalmologic examination and electrophysiologic studies were normal in all three patients. Unlike previously reported cases, visual symptoms did not resolve on cessation of treatment. Patients remain symptomatic from 2 to 7 years after discontinuing treatment with the medication.
CONCLUSIONS: Treatment with clomiphene can cause prolonged visual disturbance. Patients who develop such symptoms should be advised that continued administration may cause irreversible changes. Women with characteristic visual symptoms should be questioned about past use of clomiphene.
The most common side-effect is without question blurred vision; while this may be a problem for some Clomid users it will dissipate once use is discontinued. Beyond vision some may experience hot flashes or even abdominal discomfort but both are extremely rare as these negative effects will occur in less than 1% of those who use Clomid.
10) http://www.ncbi.nlm.nih.gov/pubmed/1761659
Hum Reprod. 1991 Aug;6(7):925-7.
Does ovarian stimulation for in-vitro fertilization induce a hypercoagulable state?
Aune B, Høie KE, Oian P, Holst N, Osterud B.
Source Department of Obstetrics and Gynaecology, University of Tromsø, Norway.
Effects on blood coagulation and fibrinolytic activity during ovarian stimulation for in-vitro fertilization (IVF) were examined in 12 women. Blood samples were taken prior to hormonal stimulation (days 2-3 of the menstrual cycle, mean serum oestradiol concentration 0.16 nmol/l) and the day after ovulation induction with human chorionic gonadotrophin (HCG) (days 10-12, mean serum oestradiol concentration 5.35 nmol/l). We measured whole blood clotting time, whole blood clot lysis time, plasma fibrinogen, factor VII and antithrombin III. The whole blood clotting time was slightly, but not significantly shortened after ovarian stimulation. A significant rise in plasma fibrinogen (P less than 0.001) and reduction in antithrombin III (P less than 0.001) were observed, whereas no change in factor VII was found. The blood fibrinolytic activity was significantly reduced as evaluated by an increase in the clot lysis time (P less than 0.02). These results indicate that ovarian stimulation for IVF may create a state of hypercoagulability.
11) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2804893/
100-fold rise in estradiol from fertility treatment increases
Thromb Res. 2009 September; 124(4): 505–507.
The effect of high circulating estradiol levels on thrombin generation during in vitro fertilization Kathleen E. Brummel-Ziedins,*‡ Matthew Gissel,* Charles Francis,† John Queenan,# and Kenneth G. Mann** Department of Biochemistry, University of Vermont, Colchester, VT, USA† Department of Medicine, University of Rochester Medical Center, Rochester, NY,
12) Image of Central Retinal Vein Occlusion
http://upload.wikimedia.org/wikipedia/commons/7/78/Branch_retinal_vein_occlusion.jpg
English: Color fundus photograph of the left eye shows occlusion of the superotemporal branch of retinal vein resulting in intraretinal hemorrhages and retinal exudates in the corresponding sector of retina.
Date 14 August 2012
Source License : http://www.biomedcentral.com/1471-2415/11/24 , Picture : http://www.biomedcentral.com/1471-2415/11/24/figure/F1
Author Ku C Yong, Tan A Kah, Yeap T Ghee, Lim C Siang and Mae-Lynn C Bastion, Department of Ophthalmology, Universiti Kebangsaan Malaysia Medical Centre (UKMMC) and Universiti Malaysia Sarawak (UNIMAS), Kuala Lumpur, Malaysia.
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
www.bioidenticalmds.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Sites and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244124.html
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
disc.yourwebapps.com/Indices/244161.html
disc.yourwebapps.com/Indices/244163.html
disc.yourwebapps.com/Indices/244163.html
health-forums.1
health-forums.2
Disclaimer click here: www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Link to this article:http://wp.me/p3gFbV-iS
Copyright (c) 2013 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of which has not always been specifically authorized by the copyright owner. We are making such material available in our efforts to advance understanding of issues of significance. We believe this constitutes a ‘fair use’ of any such copyrighted material as provided for in section 107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section 107, the material on this site is distributed without profit to those who have expressed a prior interest in receiving the included information for research and educational purposes.









Leave a Comment
You must be logged in to post a comment.